The following stereospecific synthesis is part of the scheme used by E. J. Corey of Har- vard University in the synthesis of erythronolide B, the precursor of the erythromycin antibiotics. In this remarkably simple set of reactions, the relative configurations of five chiral centers are established. OH 1. ВН, THF CrOg, H,SO4 CH;ONa 2. Н,О», NaOH acetone 2,4,6-Trimethyl- phenol HO COOH A B C Br Br Brg, H,O КОН Brg, H,O KBr THE KBr СООН E F
The following stereospecific synthesis is part of the scheme used by E. J. Corey of Har- vard University in the synthesis of erythronolide B, the precursor of the erythromycin antibiotics. In this remarkably simple set of reactions, the relative configurations of five chiral centers are established. OH 1. ВН, THF CrOg, H,SO4 CH;ONa 2. Н,О», NaOH acetone 2,4,6-Trimethyl- phenol HO COOH A B C Br Br Brg, H,O КОН Brg, H,O KBr THE KBr СООН E F
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter21: Benzene And The Concept Of Aromaticity
Section: Chapter Questions
Problem 21.55P
Related questions
Question
Account for the stereoselectivity and regioselectivity of the three steps in the conversion of compound C to compound F.
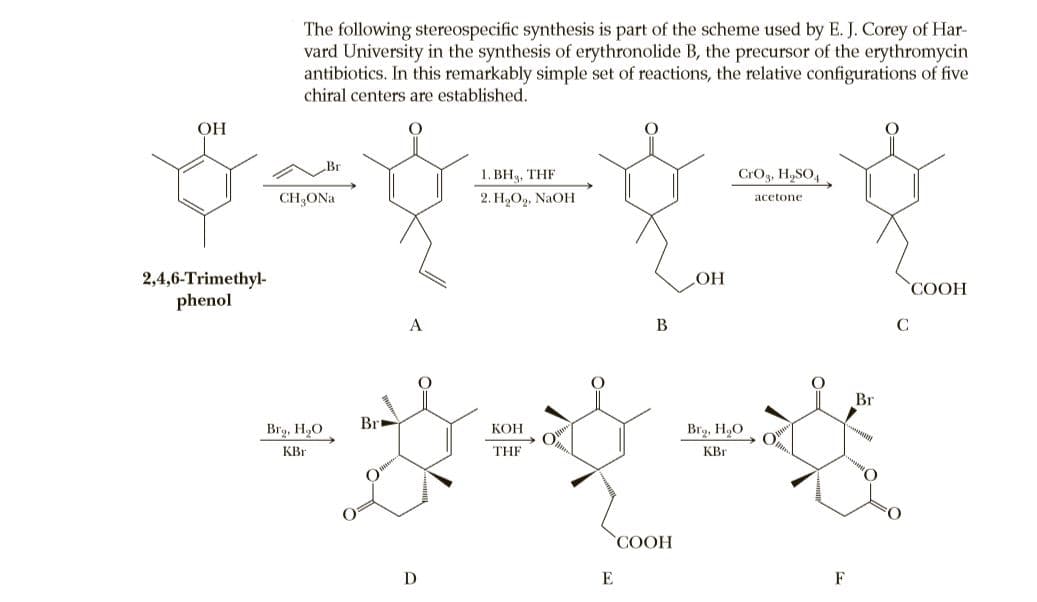
Transcribed Image Text:The following stereospecific synthesis is part of the scheme used by E. J. Corey of Har-
vard University in the synthesis of erythronolide B, the precursor of the erythromycin
antibiotics. In this remarkably simple set of reactions, the relative configurations of five
chiral centers are established.
OH
1. ВН, THF
CrOg, H,SO4
CH;ONa
2. Н,О», NaOH
acetone
2,4,6-Trimethyl-
phenol
HO
COOH
A
B
C
Br
Br
Brg, H,O
КОН
Brg, H,O
KBr
THE
KBr
СООН
E
F
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning



Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

