The forensic technician at a crime scene has just prepared a luminol stock solution by adding 15.0 g of luminol into a total volume of 75.0 mL of H3O. What is the molarity of the stock solution of luminol? Express your answer with the appropriate units. > View Available Hint(s) ? molarity of luminol solution = Value Units Submit Part B Before investigating the scene, the technician must dilute the luminol solution to a concentration of 6.00×10-2 M . The diluted solution is then placed in a spray bottle for application on the desired surfaces. How many moles of luminol are present in 2.00 L of the diluted spray? Express your answer with the appropriate units. View Availahle Hintie)
The forensic technician at a crime scene has just prepared a luminol stock solution by adding 15.0 g of luminol into a total volume of 75.0 mL of H3O. What is the molarity of the stock solution of luminol? Express your answer with the appropriate units. > View Available Hint(s) ? molarity of luminol solution = Value Units Submit Part B Before investigating the scene, the technician must dilute the luminol solution to a concentration of 6.00×10-2 M . The diluted solution is then placed in a spray bottle for application on the desired surfaces. How many moles of luminol are present in 2.00 L of the diluted spray? Express your answer with the appropriate units. View Availahle Hintie)
Chapter31: Introduction To Analytical Separations
Section: Chapter Questions
Problem 31.17QAP
Related questions
Question
Transcribed Image Text:Part A
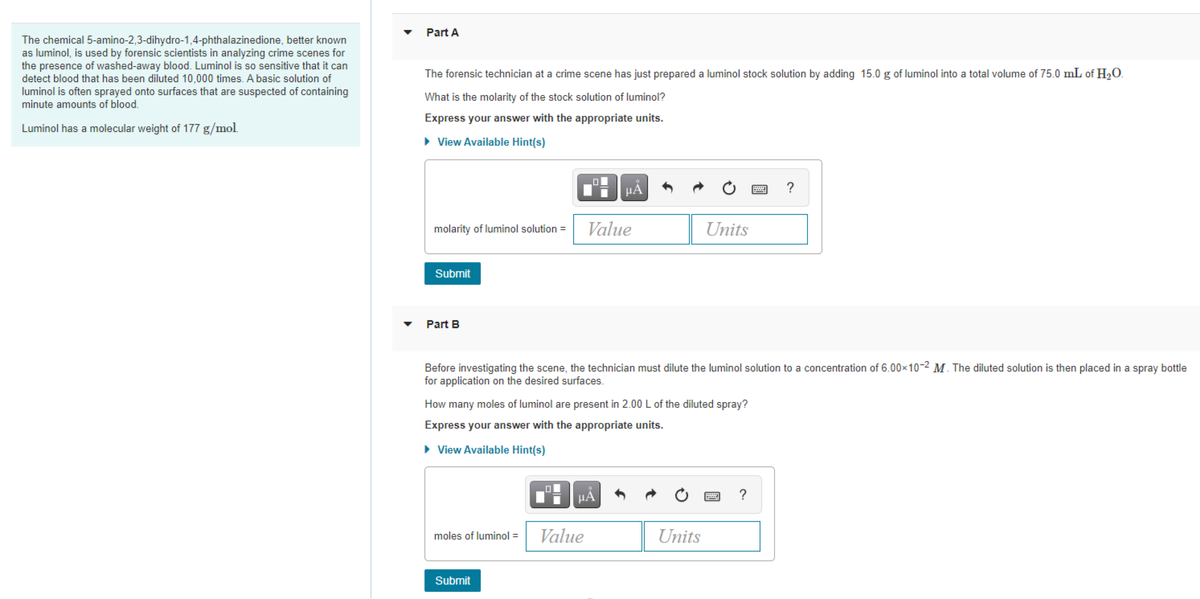
The chemical 5-amino-2,3-dihydro-1,4-phthalazinedione, better known
as luminol, is used by forensic scientists in analyzing crime scenes for
the presence of washed-away blood. Luminol is so sensitive that it can
detect blood that has been diluted 10,000 times. A basic solution of
luminol is often sprayed onto surfaces that are suspected of containing
minute amounts of blood.
The forensic technician at a crime scene has just prepared a luminol stock solution by adding 15.0 g of luminol into a total volume of 75.0 mL of H2O.
What is the molarity of the stock solution of luminol?
Express your answer with the appropriate units.
Luminol has a molecular weight of 177 g/mol.
• View Available Hint(s)
HẢ
molarity of luminol solution =
Value
Units
Submit
Part B
Before investigating the scene, the technician must dilute the luminol solution to a concentration of 6.00x10-2 M. The diluted solution is then placed in a spray bottle
for application on the desired surfaces.
How many moles of luminol are present in 2.00L of the diluted spray?
Express your answer with the appropriate units.
• View Available Hint(s)
µA
?
moles of luminol =
Value
Units
Submit
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning