The function e(x) where r = R/a (see Molecules Notes on blackboard) describes the total energy of the H molecule in units of -E1 (13.6 eV). The second derivative of ɛ(x) at the equilibrium point, can be used to estimate the natural frequency of vibration of the two protons. If the ground state energy (hw/2) of this oscillator exceeds the binding energy of the system, the protons will become unbound. Estimate the oscillator frequency w and hence the vibrational energy and show that it is small compared to the binding energy of the H, molecule. You may want to consider the following quadratic expansion of the function E(x) around the minimum e(x) - Eo + k(x – xo)² xo – 0.25 < x < xo + 0.25 2 where Eo = 1.13E1, k = -0.126E1 and ro = 2.5R/a. Note Eı is a negative constant (E1=-13.6 eV)
The function e(x) where r = R/a (see Molecules Notes on blackboard) describes the total energy of the H molecule in units of -E1 (13.6 eV). The second derivative of ɛ(x) at the equilibrium point, can be used to estimate the natural frequency of vibration of the two protons. If the ground state energy (hw/2) of this oscillator exceeds the binding energy of the system, the protons will become unbound. Estimate the oscillator frequency w and hence the vibrational energy and show that it is small compared to the binding energy of the H, molecule. You may want to consider the following quadratic expansion of the function E(x) around the minimum e(x) - Eo + k(x – xo)² xo – 0.25 < x < xo + 0.25 2 where Eo = 1.13E1, k = -0.126E1 and ro = 2.5R/a. Note Eı is a negative constant (E1=-13.6 eV)
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter20: Molecular Spectroscopy And Photochemistry
Section: Chapter Questions
Problem 14P
Related questions
Question
6.4. Answer question throughly and with much detail as possible.
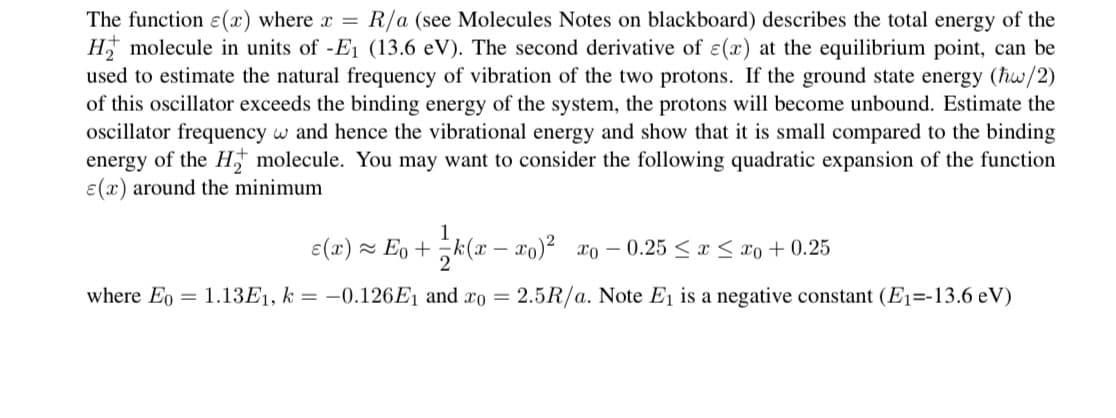
Transcribed Image Text:The function ɛ(x) where x =
H molecule in units of -E1 (13.6 eV). The second derivative of ɛ(x) at the equilibrium point, can be
used to estimate the natural frequency of vibration of the two protons. If the ground state energy (hw/2)
of this oscillator exceeds the binding energy of the system, the protons will become unbound. Estimate the
oscillator frequency w and hence the vibrational energy and show that it is small compared to the binding
energy of the H† molecule. You may want to consider the following quadratic expansion of the function
E(x) around the minimum
R/a (see Molecules Notes on blackboard) describes the total energy of the
e(x) ~ Eo + k(x – xo)² xo – 0.25 < x < xo + 0.25
2
where Eo = 1.13E1, k = –0.126E1 and xo = 2.5R/a. Note E1 is a negative constant (E1=-13.6 eV)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,