The higher the oxidation state of the chlorine atom of the chlorine atom, the more electron density it pulls from the O- H bond and the more easily the proton is lost. Sodium ethanoate is an electrolyte and ionizes completely in aqueous solution 46. HCIO, is a stronger acid than HCIO, 47. An aqueous solution of sodium ethanoate is likely to be alkaline (pH> 7) 48. Beryllium and aluminum are diagonally Beryllium and aluminium have similar electronegotivities related. 49. Pentane has a higher boiling point than n-butane.. 50. lonic compounds are usually insoluble in When an ironic compound dissolves in a polar solvent the salvation non polar solvents Pentane has more branched chain isomers than butane energy is higher then the lattice energy
The higher the oxidation state of the chlorine atom of the chlorine atom, the more electron density it pulls from the O- H bond and the more easily the proton is lost. Sodium ethanoate is an electrolyte and ionizes completely in aqueous solution 46. HCIO, is a stronger acid than HCIO, 47. An aqueous solution of sodium ethanoate is likely to be alkaline (pH> 7) 48. Beryllium and aluminum are diagonally Beryllium and aluminium have similar electronegotivities related. 49. Pentane has a higher boiling point than n-butane.. 50. lonic compounds are usually insoluble in When an ironic compound dissolves in a polar solvent the salvation non polar solvents Pentane has more branched chain isomers than butane energy is higher then the lattice energy
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter15: Solutions
Section: Chapter Questions
Problem 53QAP
Related questions
Question
show-all-working-explaining-detailly-each-step.
Answer should be typewritten using a computer keyboard
Answer Q47 & 48
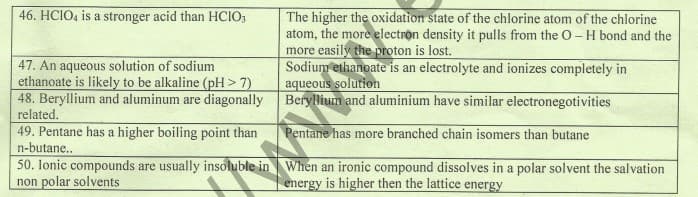
Transcribed Image Text:The higher the oxidation state of the chlorine atom of the chlorine
atom, the more electron density it pulls from the O- H bond and the
more easily the proton is lost.
Sodium ethanoate is an electrolyte and ionizes completely in
aqueous solution
46. HCIO, is a stronger acid than HCIO;
47. An aqueous solution of sodium
ethanoate is likely to be alkaline (pH > 7)
48. Beryllium and aluminum are diagonally Beryllium and aluminium have similar electronegotivities
related.
49. Pentane has a higher boiling point than
n-butane.
50. Ionic compounds are usually insoluble in When an ironic compound dissolves in a polar solvent the salvation
non polar solvents
Pentane has more branched chain isomers than butane
energy is higher then the lattice energy

Transcribed Image Text:DIRECTIONS SUMMARISED
FIRST
SECOND
STATEMΕNT
STATEMENT
A
TRUE
Second statement is a CORRECT
explanation of the tirst
Second statement is NOT a CORRECT
explanation of the first.
TRUE
TRUE
TRUE
C
TRUE
TRUE
FALSE
D
TRUE
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning