The molar absorptivity for the complex formed between bismuth(III) and thiourea is 9.32 × 103 L mol-l cm-1 at 470 nm. Calculate the range of permissible concentrations for the complex if the absorbance is to be no less than 0.15 nor greater than 0.80 when the measurements are made in 1.00-cm cells.
The molar absorptivity for the complex formed between bismuth(III) and thiourea is 9.32 × 103 L mol-l cm-1 at 470 nm. Calculate the range of permissible concentrations for the complex if the absorbance is to be no less than 0.15 nor greater than 0.80 when the measurements are made in 1.00-cm cells.
Chapter26: Molecular Absorption Spectrometry
Section: Chapter Questions
Problem 26.5QAP
Related questions
Question
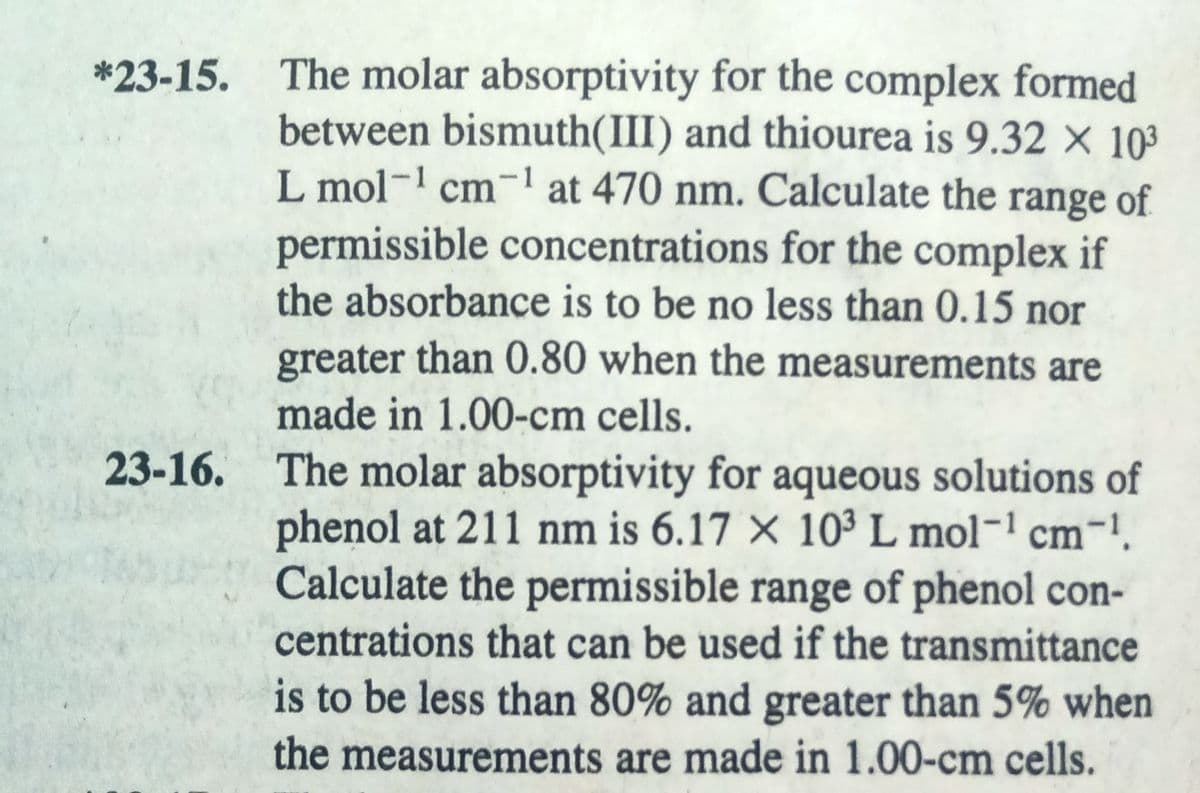
Transcribed Image Text:*23-15. The molar absorptivity for the complex formed
between bismuth(III) and thiourea is 9.32 X 10³
L mol-1 cm-1 at 470 nm. Calculate the range of
permissible concentrations for the complex if
the absorbance is to be no less than 0.15 nor
greater than 0.80 when the measurements are
made in 1.00-cm cells.
23-16. The molar absorptivity for aqueous solutions of
phenol at 211 nm is 6.17 × 10³ L mol- cm-!.
Calculate the permissible range of phenol con-
centrations that can be used if the transmittance
is to be less than 80% and greater than 5% when
the measurements are made in 1.00-cm cells.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Recommended textbooks for you



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning