The pH 6.0 buffer solution had resulted in a higher logD value than the pH 7.0 buffer solution and pH 8.0. This was because the aqueous phase at pH 7.0 was relatively more alkaline than at pH 6.0 and so more acid molecules were ionised and so partitioned more into the aqueous phase at pH 7.0. The outcome of this experiment was therefore determining that benzoic acid is more lipophilic at pH 6.0 than at pH 7.0 and pH 8.0.
The pH 6.0 buffer solution had resulted in a higher logD value than the pH 7.0 buffer solution and pH 8.0. This was because the aqueous phase at pH 7.0 was relatively more alkaline than at pH 6.0 and so more acid molecules were ionised and so partitioned more into the aqueous phase at pH 7.0. The outcome of this experiment was therefore determining that benzoic acid is more lipophilic at pH 6.0 than at pH 7.0 and pH 8.0.
Chapter9: Parenteral Medication Labels And Dosage Calculation
Section: Chapter Questions
Problem 4.1P
Related questions
Question
100%
Hi, i dont understand the steps here. Could you rewrite this in a more simple manner so i could understand better.
Thanks
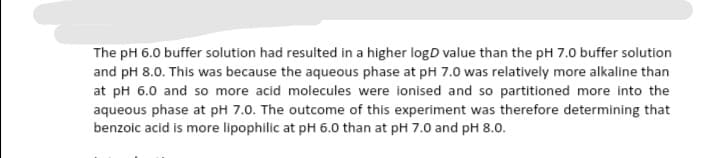
Transcribed Image Text:The pH 6.0 buffer solution had resulted in a higher logD value than the pH 7.0 buffer solution
and pH 8.0. This was because the aqueous phase at pH 7.0 was relatively more alkaline than
at pH 6.0 and so more acid molecules were ionised and so partitioned more into the
aqueous phase at pH 7.0. The outcome of this experiment was therefore determining that
benzoic acid is more lipophilic at pH 6.0 than at pH 7.0 and pH 8.0.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

