The powerful electron-withdrawing property of a carbonyl group increases the acidity of a-protons (i.e., protons attached to a carbon that is adjacent to the carbonyl group). The effect varies depending on what else is attached to the carbonyl, and whether there is a second carbonyl group attached to the a-carbon. Rank the structures from highest acidity (lowest pKa value) to lowest acidity (highest pK, value). The acidic hydrogen is indicated in blue in each structure.
The powerful electron-withdrawing property of a carbonyl group increases the acidity of a-protons (i.e., protons attached to a carbon that is adjacent to the carbonyl group). The effect varies depending on what else is attached to the carbonyl, and whether there is a second carbonyl group attached to the a-carbon. Rank the structures from highest acidity (lowest pKa value) to lowest acidity (highest pK, value). The acidic hydrogen is indicated in blue in each structure.
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter25: Enolate & Enol Nucleophiles
Section: Chapter Questions
Problem 4E
Related questions
Question
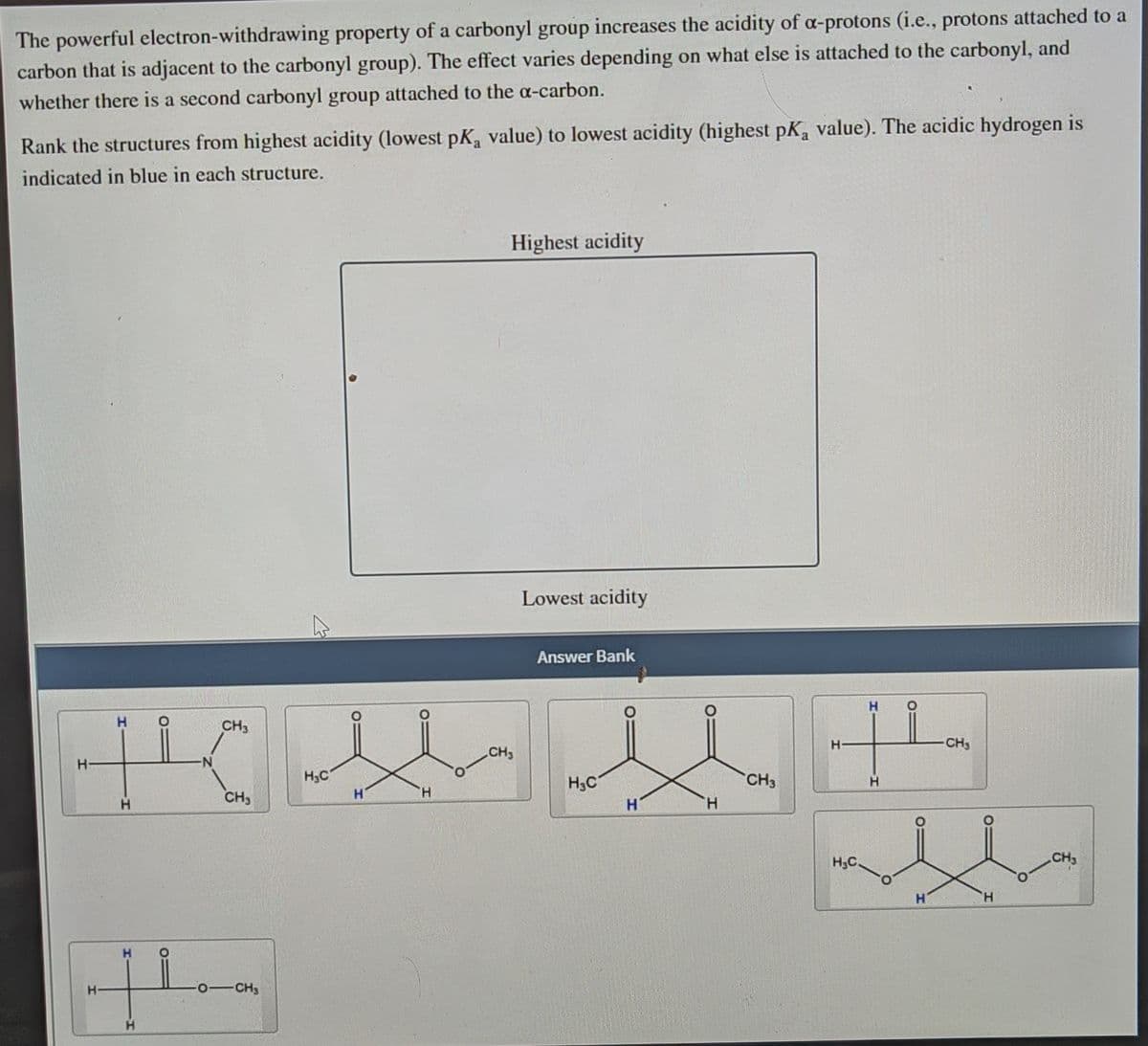
Transcribed Image Text:The powerful electron-withdrawing property of a carbonyl group increases the acidity of a-protons (i.e., protons attached to a
carbon that is adjacent to the carbonyl group). The effect varies depending on what else is attached to the carbonyl, and
whether there is a second carbonyl group attached to the a-carbon.
Rank the structures from highest acidity (lowest pK, value) to lowest acidity (highest pK, value). The acidic hydrogen is
indicated in blue in each structure.
Highest acidity
Lowest acidity
Answer Bank
H.
H.
CH3
H.
-CH
CH3
H.C
H.
H3C
CH3
H.
H.
CH
H.
H,C
CH3
H.
H.
H.
-CH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning
