The rate constant of the hydrolysis of ethyl acetate (conc = 0.08 M) by sodium hydroxide (conc = 0.08 M) was determined by monitoring the conductance of the solution over time. Determine the rate constant and briefly discuss how you were able to solve for it.
The rate constant of the hydrolysis of ethyl acetate (conc = 0.08 M) by sodium hydroxide (conc = 0.08 M) was determined by monitoring the conductance of the solution over time. Determine the rate constant and briefly discuss how you were able to solve for it.
Chapter13: Kinetic Methods
Section: Chapter Questions
Problem 4P
Related questions
Question
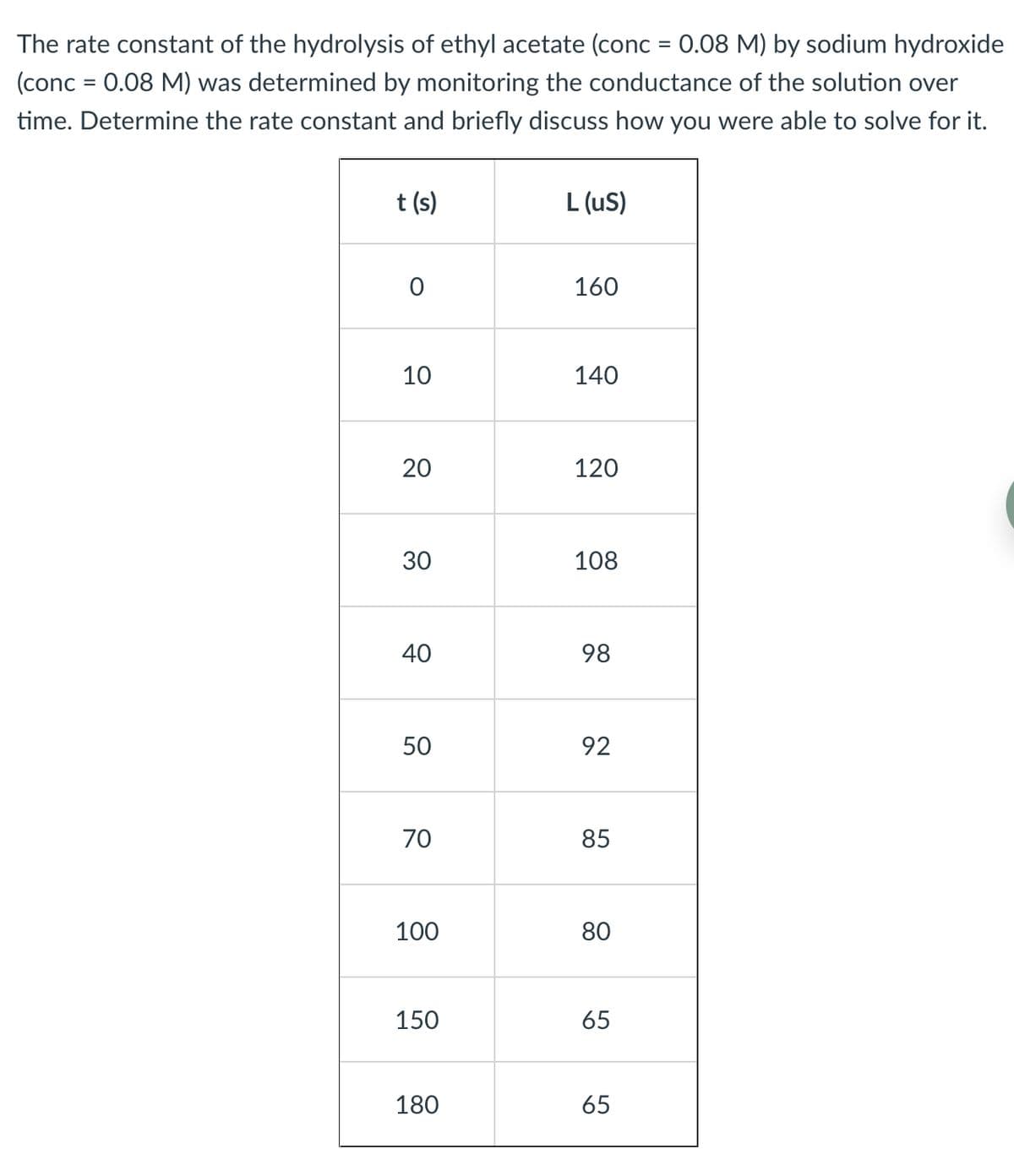
Transcribed Image Text:The rate constant of the hydrolysis of ethyl acetate (conc = 0.08 M) by sodium hydroxide
(conc = 0.08 M) was determined by monitoring the conductance of the solution over
time. Determine the rate constant and briefly discuss how you were able to solve for it.
t (s)
L (uS)
160
10
140
20
120
30
108
40
98
50
92
70
85
100
80
150
65
180
65
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 1 images

Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
