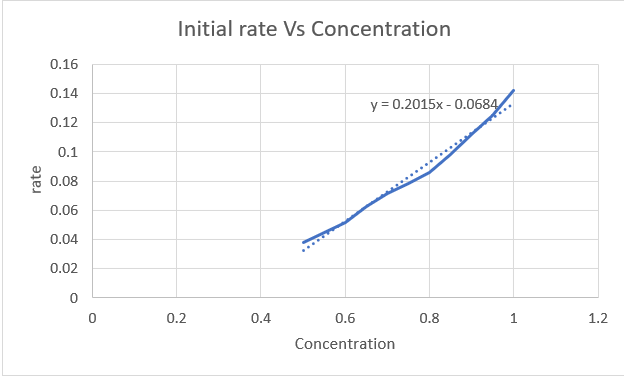
The rate law for the decomposition of A→P is investigated by measuring the initial rate of the reaction for several concentrations of A. The data are shown below. Assuming that the rate law is of the form v=k[A]n, use a graphical method to determine the value of n. [A]/M Initial Rate/(Torr s-1) [A]/M Initial Rate/(Torr s-1) 1.000 0.1422 0.700 0.0718 0.950 0.1248 0.650 0.0625 0.900 0.1123 0.600 0.0518 0.850 0.0983 0.550 0.0445 0.800 0.0857 0.500 0.0382 0.750 0.0782
The rate law for the decomposition of A→P is investigated by measuring the initial rate of the reaction for several concentrations of A. The data are shown below. Assuming that the rate law is of the form v=k[A]n, use a graphical method to determine the value of n. [A]/M Initial Rate/(Torr s-1) [A]/M Initial Rate/(Torr s-1) 1.000 0.1422 0.700 0.0718 0.950 0.1248 0.650 0.0625 0.900 0.1123 0.600 0.0518 0.850 0.0983 0.550 0.0445 0.800 0.0857 0.500 0.0382 0.750 0.0782
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter14: Chemical Kinetics: The Rates Of Chemical Reactions
Section: Chapter Questions
Problem 86IL: The acid-catalyzed iodination of acetone CH3COCH3(aq) + I2(aq) CH3COCH2I(aq) + HI(aq) is a common...
Related questions
Question
- The rate law for the decomposition of A→P is investigated by measuring the initial
rate of the reaction for several concentrations of A. The data are shown below. Assuming that the rate law is of the form v=k[A]n, use a graphical method to determine the value of n.
|
[A]/M |
Initial Rate/(Torr s-1) |
[A]/M |
Initial Rate/(Torr s-1) |
|
1.000 |
0.1422 |
0.700 |
0.0718 |
|
0.950 |
0.1248 |
0.650 |
0.0625 |
|
0.900 |
0.1123 |
0.600 |
0.0518 |
|
0.850 |
0.0983 |
0.550 |
0.0445 |
|
0.800 |
0.0857 |
0.500 |
0.0382 |
|
0.750 |
0.0782 |
|
|
Expert Solution
Step 1
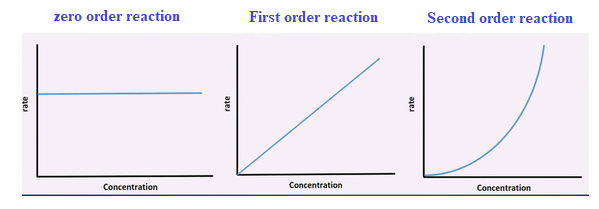
Graphs are plotted of rate of reaction against concentration.
If it is a straight line the reaction is first order reaction.
A line which is independent of concentration is zero order reaction.
If a curve is obtained, then it is second order reaction.
Step 2
Plot of rate Vs concentration
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,