The rate of a certain reaction was studied at various T(K) k (s temperatures. The table shows temperature (T) and rate 400 0.000278 constant (k) data collected during the experiments. Plot the 420 0.00189 data to answer the questions. 440 0.0108 What is the value of the activation energy, Ea, for 460 0.0532 this reaction? 480 0.229 500 0.878 520 3.03 -1 Ea = kJ • mol 540 9.56 560 27.8 580 74.9 What is the value of the pre-exponential factor (sometimes called the frequency factor), A, for this reaction? A = s-1
The rate of a certain reaction was studied at various T(K) k (s temperatures. The table shows temperature (T) and rate 400 0.000278 constant (k) data collected during the experiments. Plot the 420 0.00189 data to answer the questions. 440 0.0108 What is the value of the activation energy, Ea, for 460 0.0532 this reaction? 480 0.229 500 0.878 520 3.03 -1 Ea = kJ • mol 540 9.56 560 27.8 580 74.9 What is the value of the pre-exponential factor (sometimes called the frequency factor), A, for this reaction? A = s-1
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter18: Chemical Kinetics
Section: Chapter Questions
Problem 45P
Related questions
Concept explainers
Bond Parameters
Many factors decide the covalent bonding between atoms. Some of the bond parameters are bond angle, bond order, enthalpy, bond length, etc. These parameters decide what kind of bond will form in atoms. Hence it is crucial to understand these parameters in detail and understand how changing these parameters affects the kind of bonding or various characteristics.
Bond Dissociation Energy
The tendency of an atom to attract an electron is known as its electronegativity.
Question
use chart provided
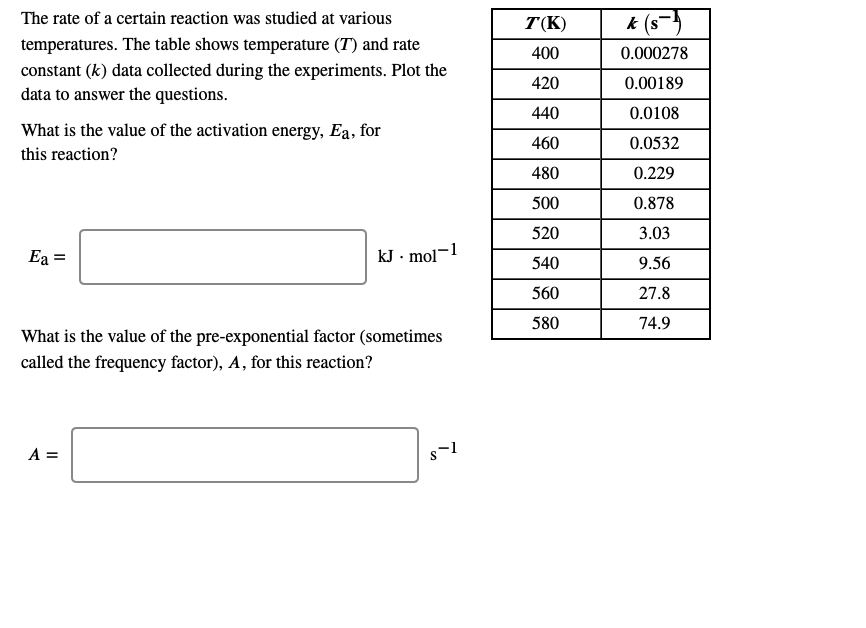
Transcribed Image Text:The rate of a certain reaction was studied at various
T(K)
k (s
temperatures. The table shows temperature (T) and rate
constant (k) data collected during the experiments. Plot the
400
0.000278
420
0.00189
data to answer the questions.
440
0.0108
What is the value of the activation energy, Ea, for
460
0.0532
this reaction?
480
0.229
500
0.878
520
3.03
Ea =
kJ · mol-1
540
9.56
560
27.8
580
74.9
What is the value of the pre-exponential factor (sometimes
called the frequency factor), A, for this reaction?
A =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole