The rate of a certain reaction was studied at various T(K) k (s-1) temperatures. The table shows temperature (T) and rate 400 0.000659 constant (k) data collected during the experiments. Plot the 420 0.00327 data to answer the questions. 440 0.0181 What is the value of the activation energy, Ea, for 460 0.0589 this reaction? 480 0.213 500 0.615 520 1.66 540 3.99 E = kJ · mol-! 560 9.64 580 20.1 What is the value of the pre-exponential factor (sometimes called the frequency factor), A, for this reaction? A =
The rate of a certain reaction was studied at various T(K) k (s-1) temperatures. The table shows temperature (T) and rate 400 0.000659 constant (k) data collected during the experiments. Plot the 420 0.00327 data to answer the questions. 440 0.0181 What is the value of the activation energy, Ea, for 460 0.0589 this reaction? 480 0.213 500 0.615 520 1.66 540 3.99 E = kJ · mol-! 560 9.64 580 20.1 What is the value of the pre-exponential factor (sometimes called the frequency factor), A, for this reaction? A =
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter20: Kinetics
Section: Chapter Questions
Problem 20.44E
Related questions
Question
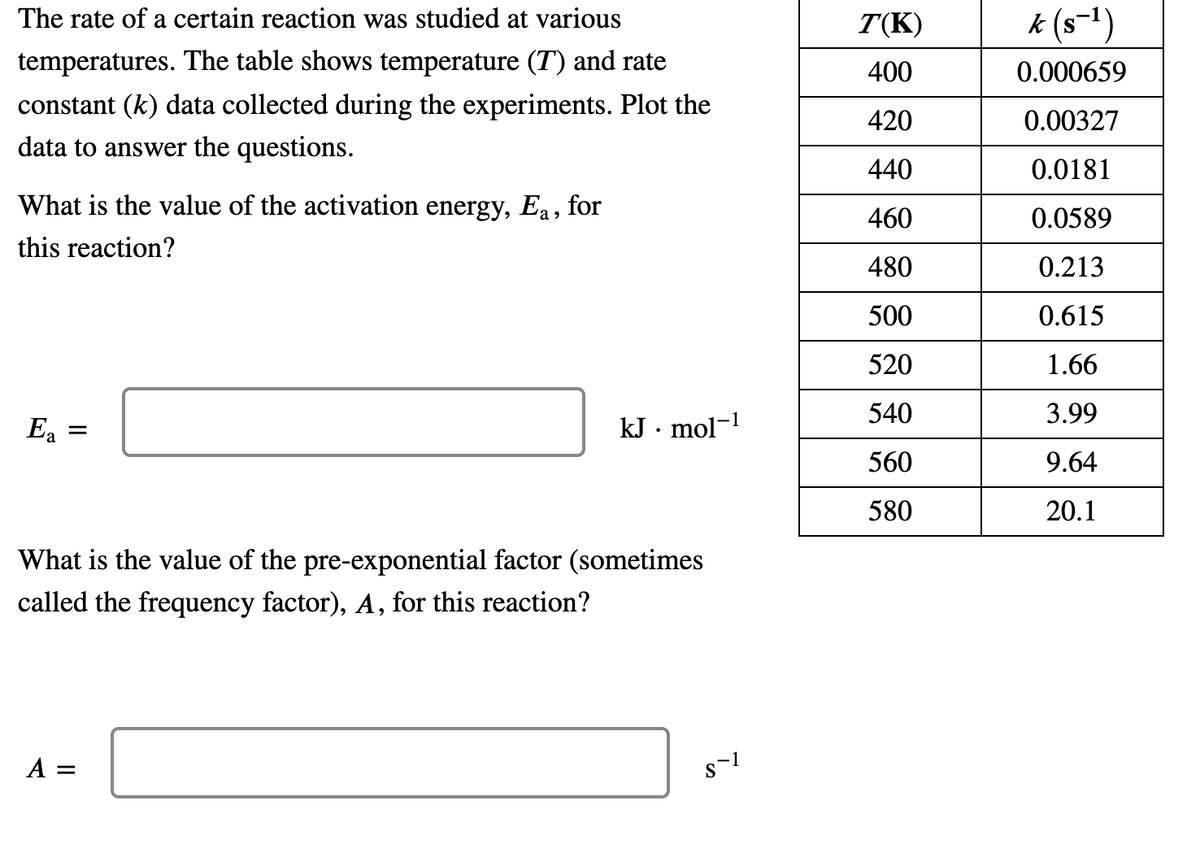
Transcribed Image Text:The rate of a certain reaction was studied at various
T(K)
k (s-1)
temperatures. The table shows temperature (T) and rate
400
0.000659
constant (k) data collected during the experiments. Plot the
420
0.00327
data to answer the questions.
440
0.0181
What is the value of the activation energy, Ea, for
460
0.0589
this reaction?
480
0.213
500
0.615
520
1.66
540
3.99
Ea
kJ · mol-
560
9.64
580
20.1
What is the value of the pre-exponential factor (sometimes
called the frequency factor), A, for this reaction?
A =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
