The rate of the reaction in terms of the "appoarance of product" includes the change in the concentration of the product, the time interval, and the coefficient of the product. Consider the following reaction: 2A + 3B-3C + 2D The concentrations of product C at three different time intervals are given. Use the following data to determine the rate of reaction in terms of the appearance of product C between time = 0s and time = 20 s. Time (s) o 20 C(M) 0.000 0.0480 0.0960 40 Express your answer in molar concentration per second to three significant figures. • View Available Hint(s) ? The rate of reaction = M -s 1
The rate of the reaction in terms of the "appoarance of product" includes the change in the concentration of the product, the time interval, and the coefficient of the product. Consider the following reaction: 2A + 3B-3C + 2D The concentrations of product C at three different time intervals are given. Use the following data to determine the rate of reaction in terms of the appearance of product C between time = 0s and time = 20 s. Time (s) o 20 C(M) 0.000 0.0480 0.0960 40 Express your answer in molar concentration per second to three significant figures. • View Available Hint(s) ? The rate of reaction = M -s 1
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter14: Chemical Kinetics: The Rates Of Chemical Reactions
Section: Chapter Questions
Problem 6PS: 6. Phenyl acetate, an ester, reacts with water according to the equation
The data in the table were...
Related questions
Question
Question in image
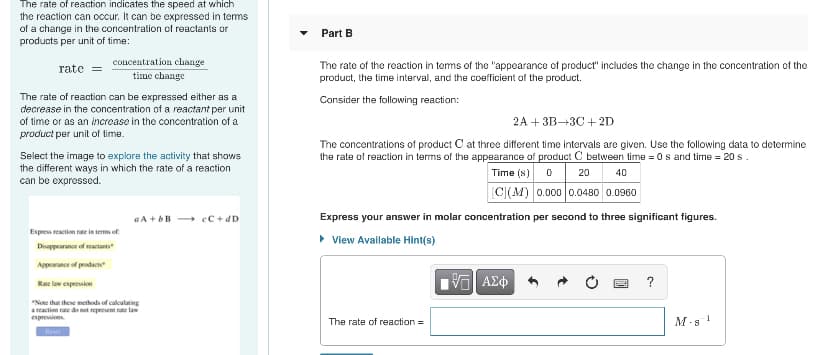
Transcribed Image Text:The rate of reaction indicates the speed at which
the reaction can occur. It can be expressed in temms
of a change in the concentration of reactants or
products per unit of time:
Part B
concentration change
The rate of the reaction in terms of the "appearance of product" includes the change in the concentration of the
product, the time interval, and the coefficient of the product.
rate
time change
The rate of reaction can be expressed either as a
decrease in the concentration of a reactant per unit
of time or as an increase in the concentration of a
Consider the following reaction:
2A + 3B→3C + 2D
product per unit of time.
The concentrations of product C at three different time intervals are given. Use the following data to determine
the rate of reaction in terms of the appearance of product C between time = 0s and time = 20 s.
Select the image to explore the activity that shows
the different ways in which the rate of a reaction
can be exprossed.
Time (s) o
20
40
[C|(M) 0.000 0.0480 0.0960
a A + bB cC + dD
Express your answer in molar concentration per second to three significant figures.
Express reaction rate in term of
• View Avallable Hint(s)
Disappearance of reactants
Appearance of prodact
Rae law expresion
"Note that these methods of caleulating
a reaction rate do net represent rate law
espresiens
The rate of reaction =
M-s !
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning