The rate of the reaction in terms of the "appearance of product" includes the change in the concentration of the product, the time interval, and the coefficient of the product. Consider the following reaction: 2A + 3B→3C+ 2D The concentrations of product C at three different time intervals are given. Use the following data to determine the rate of reaction in terms of the appearance of product C between time = 0 s and time = 20 s . Time (s) 20 40 C(M) 0.000 0.0120 0.0240 Express your answer in molar concentration per second to three significant figures. • View Available Hint(s) ν ΑΣΦΦ The rate of reaction = M·s-1
The rate of the reaction in terms of the "appearance of product" includes the change in the concentration of the product, the time interval, and the coefficient of the product. Consider the following reaction: 2A + 3B→3C+ 2D The concentrations of product C at three different time intervals are given. Use the following data to determine the rate of reaction in terms of the appearance of product C between time = 0 s and time = 20 s . Time (s) 20 40 C(M) 0.000 0.0120 0.0240 Express your answer in molar concentration per second to three significant figures. • View Available Hint(s) ν ΑΣΦΦ The rate of reaction = M·s-1
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter14: Chemical Kinetics: The Rates Of Chemical Reactions
Section: Chapter Questions
Problem 12PS: The reaction 2 NO(g) + 2 H2(g) N2(g) + 2 H2O(g) was studied at 904 C, and the data in the table...
Related questions
Question
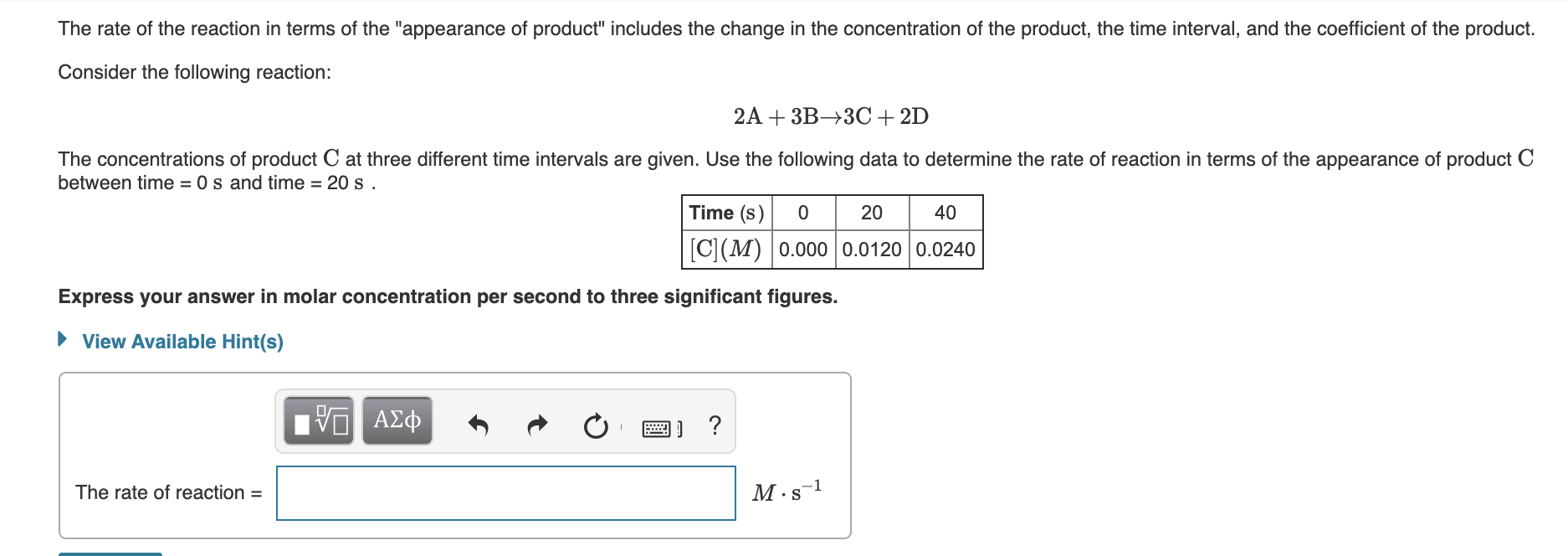
Transcribed Image Text:The rate of the reaction in terms of the "appearance of product" includes the change in the concentration of the product, the time interval, and the coefficient of the product.
Consider the following reaction:
2A + 3B→3C+ 2D
The concentrations of product C at three different time intervals are given. Use the following data to determine the rate of reaction in terms of the appearance of product C
between time = 0 s and time = 20 s .
Time (s)
20
40
C(M) 0.000 0.0120 0.0240
Express your answer in molar concentration per second to three significant figures.
• View Available Hint(s)
ν ΑΣΦΦ
The rate of reaction =
M·s-1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning