The reaction of methane with water to form carbon dioxide and hydrogen is non- spontaneous at 298 K. At what temperature will this system make the transition from non-spontaneous to spontaneous? The following data is valid at 298 K: CH4(g) + 2H2O(g) = Substance: CH4(8) CO2(8) + 4H2(g) H20(g) CO2(3) H2(g) -74.87 -241.8 -393.5 (kJ/mol): AG (kJ/mol): S(J/K • mol): -50.81 -228.6 -394.4 186.1 188.8 213.7 130.7 HINT: Calculate AHxn. A So°, then determine T, must show work, write final answers below, upload work
The reaction of methane with water to form carbon dioxide and hydrogen is non- spontaneous at 298 K. At what temperature will this system make the transition from non-spontaneous to spontaneous? The following data is valid at 298 K: CH4(g) + 2H2O(g) = Substance: CH4(8) CO2(8) + 4H2(g) H20(g) CO2(3) H2(g) -74.87 -241.8 -393.5 (kJ/mol): AG (kJ/mol): S(J/K • mol): -50.81 -228.6 -394.4 186.1 188.8 213.7 130.7 HINT: Calculate AHxn. A So°, then determine T, must show work, write final answers below, upload work
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter16: Thermodynamics
Section: Chapter Questions
Problem 61E: The evaporation of one mole of water at 298 K has a standard free allergy change of 8.58 kJ....
Related questions
Question

Transcribed Image Text:The reaction of methane with water to form carbon dioxide and hydrogen is non-
spontaneous at 298 K. At what temperature will this system make the transition
from non-spontaneous to spontaneous? The following data is valid at 298 K:
CHĄ(g) + 2H2O(g) =CO2(g) + 4H2(g)
H2O(g)
Substance: CH4(8)
CO2(3)
H2(g)
-74.87
-241.8
-393.5
(kJ/mol):
AGf
-50.81
-228.6
-394.4
(kJ/mol):
S(J/K •
mol):
186.1
188.8
213.7
130.7
HINT: Calculate AH pxn. A So, then determine T, must show work, write final answers
below, upload work.
Expert Solution
Step 1
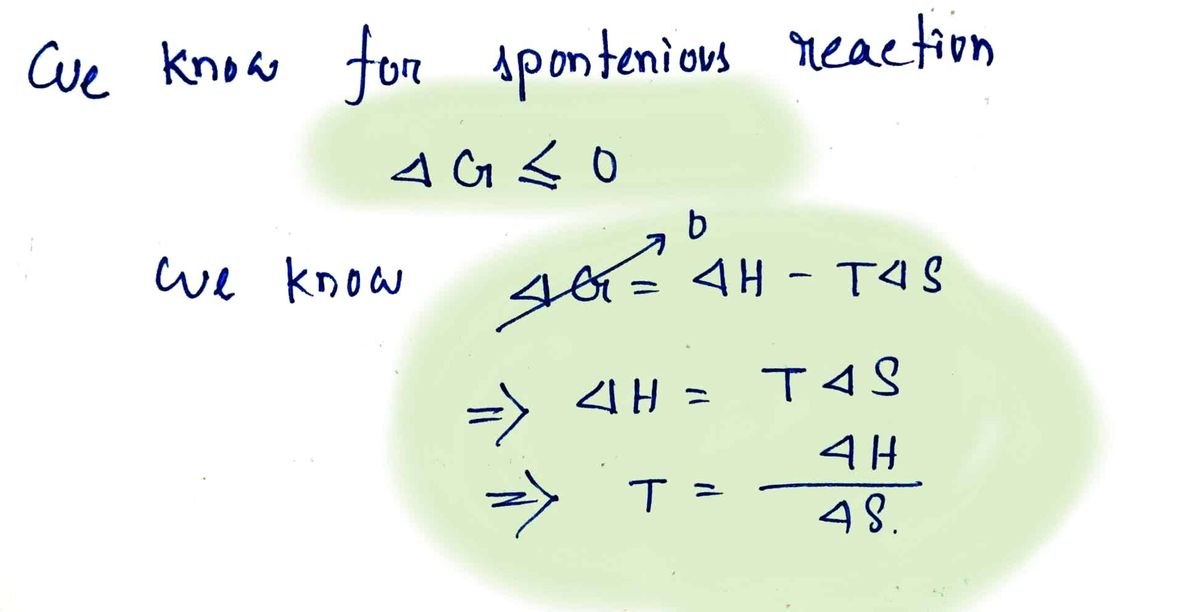
To become the spontaneous reaction it must have change in Gibbs free energy negative. In order to do so we have the formulas to calculate temperature.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning