The relative displacement (r/ro) of bovine serum albumin was observed as a function of time: t (s) 700 3580 4540 5020 r/r. 1.0129 1.0679 1.0871 1.0965 Given w = 6 260 /s, find the sedimentation coefficient. Assuming v = 7.34x 10 3 m3 kg and p = 9.93 x 102 kg/m3, diffusivity = 6.97 x 10-11 m2/s at 25 °C, determine the molar %3D %3D mass of the sample in kg/mol.
The relative displacement (r/ro) of bovine serum albumin was observed as a function of time: t (s) 700 3580 4540 5020 r/r. 1.0129 1.0679 1.0871 1.0965 Given w = 6 260 /s, find the sedimentation coefficient. Assuming v = 7.34x 10 3 m3 kg and p = 9.93 x 102 kg/m3, diffusivity = 6.97 x 10-11 m2/s at 25 °C, determine the molar %3D %3D mass of the sample in kg/mol.
Chapter34: Miscellaneous Separation Methods
Section: Chapter Questions
Problem 34.15QAP
Related questions
Question
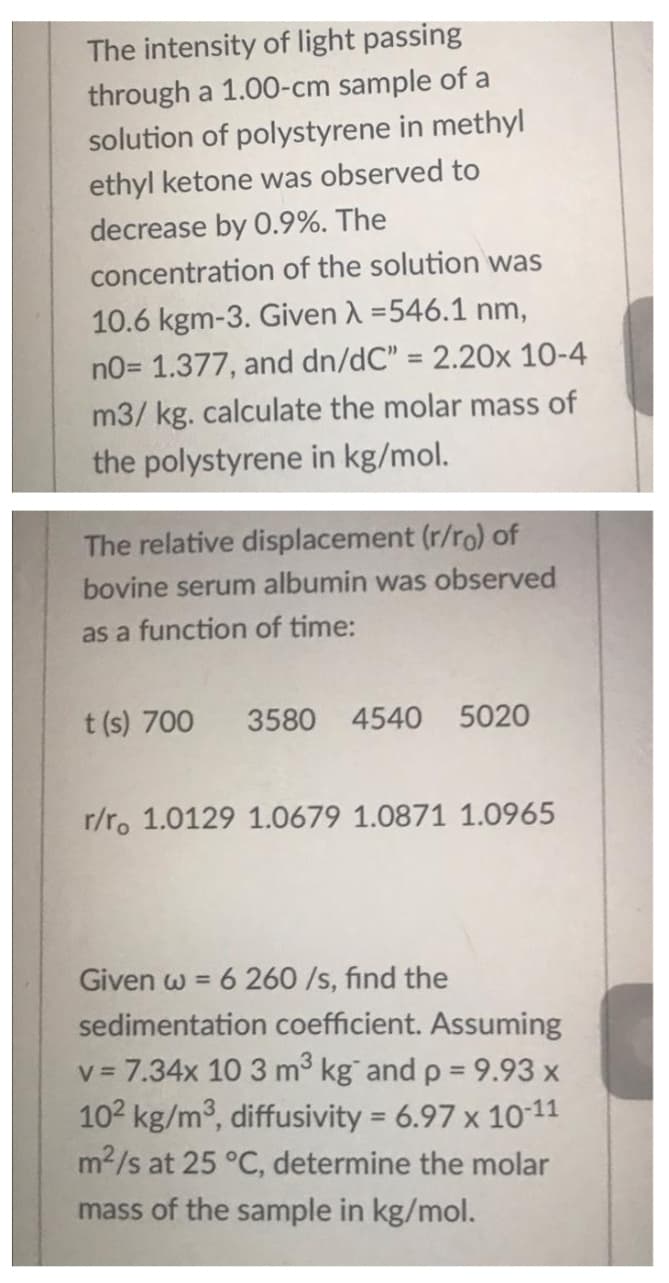
Transcribed Image Text:The intensity of light passing
through a 1.00-cm sample of a
solution of polystyrene in methyl
ethyl ketone was observed to
decrease by O.9%. The
concentration of the solution was
10.6 kgm-3. Given A =546.1 nm,
no= 1.377, and dn/dC" = 2.20x 10-4
%3D
m3/ kg. calculate the molar mass of
the polystyrene in kg/mol.
The relative displacement (r/ro) of
bovine serum albumin was observed
as a function of time:
t (s) 700
3580 4540 5020
r/r. 1.0129 1.0679 1.0871 1.0965
6 260 /s, find the
sedimentation coefficient. Assuming
Given w =
v = 7.34x 10 3 m3 kg and p = 9.93 x
102 kg/m3, diffusivity = 6.97 x 1011
m2/s at 25 °C, determine the molar
%3D
mass of the sample in kg/mol.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning