The total pressure of the following system is used to monitor the progress of the chemical reaction. SO₂Cl(g) → SO(g) + Cla(g) The reaction is initiated, and the following data are obtained: 0 3 6 Time (h) 9 12 15 20.71 Potal (kPa) 11.07 14.79 17.26 18.90 19.99 Pso₂Cl₂ Pso₂ Pcl₂ Additional information: As the reaction proceeds, stoichiometry dictates that for every mole of SO,Cl; that dissociates one mole of each of SO₂ and Cl₂ is produced. Defining the extent of dissociation/reaction as , and the initial pressure as Po, the total pressure is given by: Ptotal= Pso,cl, + Pso,+Pa,= (Po-5)+5+5 a) Calculate the pressure of each component at each time interval, i.e. complete the above table. b) Use a graphical method and determine what the order of the reaction is with respect to SO,Cl₂? (i) Show the plot as well as the linear fit of the data. (ii) Motivate your answer. c) Determine the rate constant for this reaction.
The total pressure of the following system is used to monitor the progress of the chemical reaction. SO₂Cl(g) → SO(g) + Cla(g) The reaction is initiated, and the following data are obtained: 0 3 6 Time (h) 9 12 15 20.71 Potal (kPa) 11.07 14.79 17.26 18.90 19.99 Pso₂Cl₂ Pso₂ Pcl₂ Additional information: As the reaction proceeds, stoichiometry dictates that for every mole of SO,Cl; that dissociates one mole of each of SO₂ and Cl₂ is produced. Defining the extent of dissociation/reaction as , and the initial pressure as Po, the total pressure is given by: Ptotal= Pso,cl, + Pso,+Pa,= (Po-5)+5+5 a) Calculate the pressure of each component at each time interval, i.e. complete the above table. b) Use a graphical method and determine what the order of the reaction is with respect to SO,Cl₂? (i) Show the plot as well as the linear fit of the data. (ii) Motivate your answer. c) Determine the rate constant for this reaction.
Chapter17: Spontaneity, Entropy, And Free Energy
Section: Chapter Questions
Problem 23Q: Monochloroethane (C2H5Cl) can be produced by the direct reaction of ethane gas (C2H6) with chlorine...
Related questions
Question
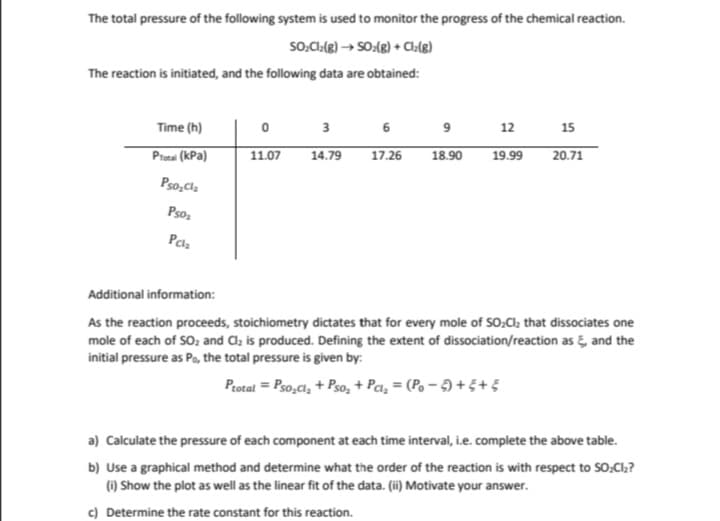
Transcribed Image Text:The total pressure of the following system is used to monitor the progress of the chemical reaction.
SO₂Cl2(g) → SO2(g) + Cl.(g)
The reaction is initiated, and the following data are obtained:
3
6
0
9
12
Time (h)
15
17.26
18.90
19.99
20.71
PTotal (kPa)
11.07
14.79
Pso₂Cl₂
Pso₂
Pcl₂
Additional information:
As the reaction proceeds, stoichiometry dictates that for every mole of SO₂Cl₂ that dissociates one
mole of each of SO₂ and Cl₂ is produced. Defining the extent of dissociation/reaction as, and the
initial pressure as Po, the total pressure is given by:
Ptotal = Pso₂Cl₂ + Pso₂+ Pal₂ = (Po-5)+5+5
a) Calculate the pressure of each component at each time interval, i.e. complete the above table.
b) Use a graphical method and determine what the order of the reaction is with respect to SO,Cl₂?
(i) Show the plot as well as the linear fit of the data. (ii) Motivate your answer.
c) Determine the rate constant for this reaction.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 6 steps with 2 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
Give the rate equation describing the reaction. How long will the reaction take to come to an end. Show all calculations or derivation of the rate equation.
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning