The vapour pressure of a substance describes how readily molecules at the surface of the substance enter the gaseous phase. At the boiling point of a liquid, the liquid's vapour pressure is equal to or greater than the atmospheric pressure exerted on the surface of the liquid. Since the atmospheric pressure at higher elevations is lower than at sea level, the boiling point of water decreases as the elevation increases. The atmospheric pressure at sea level is 760 mmHg. This pressure decreases by 6.50 mmHg for every 100 m increase in elevation. Part A What is the boiling point of water at an elevation of 6000 m ? Express your answer with the appropriate units using three significant figures. Elevation Pressure > View Available Hint(s) 0m 760 mmHg 695 mmHg Peset Tempjetes Symbols uhdo redo 1000 m keyboard shortcuts Help 2000 m 630 mmHg T = 94.3 The boiling point of water decreases 0.05 °C for every 1 mmHg drop in atmospheric pressure
The vapour pressure of a substance describes how readily molecules at the surface of the substance enter the gaseous phase. At the boiling point of a liquid, the liquid's vapour pressure is equal to or greater than the atmospheric pressure exerted on the surface of the liquid. Since the atmospheric pressure at higher elevations is lower than at sea level, the boiling point of water decreases as the elevation increases. The atmospheric pressure at sea level is 760 mmHg. This pressure decreases by 6.50 mmHg for every 100 m increase in elevation. Part A What is the boiling point of water at an elevation of 6000 m ? Express your answer with the appropriate units using three significant figures. Elevation Pressure > View Available Hint(s) 0m 760 mmHg 695 mmHg Peset Tempjetes Symbols uhdo redo 1000 m keyboard shortcuts Help 2000 m 630 mmHg T = 94.3 The boiling point of water decreases 0.05 °C for every 1 mmHg drop in atmospheric pressure
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter10: Solids, Liquids, And Phase Transitions
Section: Chapter Questions
Problem 69AP
Related questions
Question
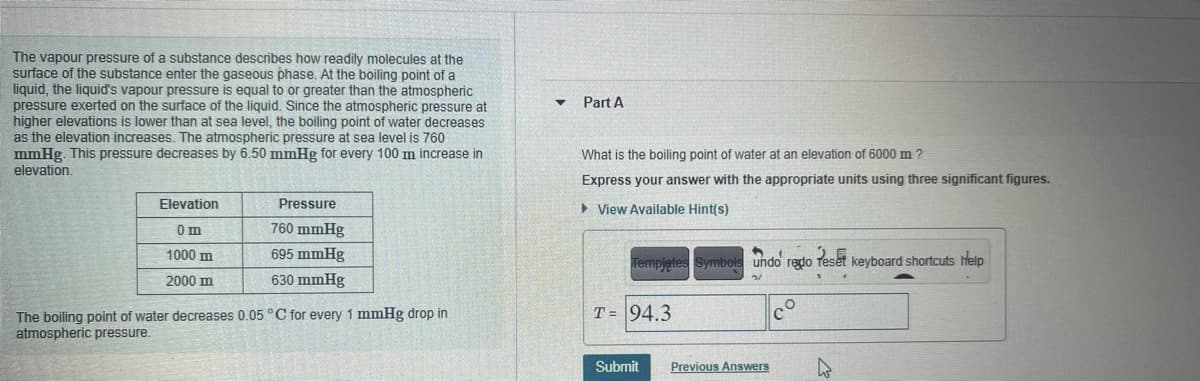
Transcribed Image Text:The vapour pressure of a substance describes how readily molecules at the
surface of the substance enter the gaseous phase. At the boiling point of a
liquid, the liquid's vapour pressure is equal to or greater than the atmospheric
pressure exerted on the surface of the liquid. Since the atmospheric pressure at
higher elevations is lower than at sea level, the boiling point of water decreases
as the elevation increases. The atmospheric pressure at sea level is 760
mmHg. This pressure decreases by 6.50 mmHg for every 100 m increase in
elevation.
Part A
What is the boiling point of water at an elevation of 6000 m ?
Express your answer with the appropriate units using three significant figures.
Elevation
Pressure
> View Available Hint(s)
Om
760 mmHg
1000 m
695 mmHg
Templetes Symbols undo redo
Peset
keyboard shortcuts help
2000 m
630 mmHg
T = 94.3
The boiling point of water decreases 0.05 C for every 1 mmHg drop in
atmospheric pressure.
Submit
Previous Answers
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT