Theoretical Yield: Stubo, Soutasqmst moon en 21 cut162 • moles of acetic anhydride = 1ml = 0.0098 mo Now nature bit to N71102.09 g/mol OCTU02 • moles of Salisylic acid = 3.00 x 10-3 mol 7 •theoretical yield = (0.002995 mois) (0.0105 mois (3.00 × 1073) (0.0098) = 0.540 grams 10°
Theoretical Yield: Stubo, Soutasqmst moon en 21 cut162 • moles of acetic anhydride = 1ml = 0.0098 mo Now nature bit to N71102.09 g/mol OCTU02 • moles of Salisylic acid = 3.00 x 10-3 mol 7 •theoretical yield = (0.002995 mois) (0.0105 mois (3.00 × 1073) (0.0098) = 0.540 grams 10°
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter20: Organic Chemistry
Section: Chapter Questions
Problem 28E: Acetylene is a very weak acid; however, it will react with moist silver(I) oxide and form water and...
Related questions
Question
100%
Can someone help me out here? There first time I calculated the theoretical yield for this experiment I got 0.540g with the given values but I actually used the wrong values. I added the new values above and the units used are mols. I can't seem to figure out or remember how I calculated 0.540g with the (3.00x10^-3 mols)(0.0098 mols). Can someone please help me by telling me how to get grams by using the equation (0.002995 mols)(0.0105mols)? I know the value should be relatively close to 0.540g but every time I try to calculate it, my answer is no where near 0.540g.
Please answer clearly and concisely. Also please make sure to read the question carefully, thank you!
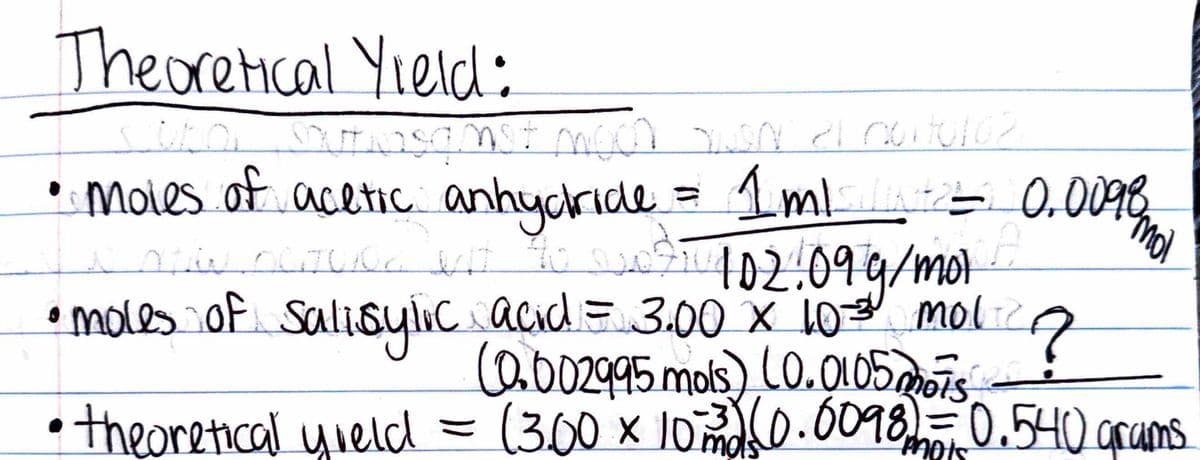
Transcribed Image Text:Theoretical Yield:
Stubo, Soutasqmst moon en 21 cut162
• moles of acetic anhydride
= 1ml
= 0.0098 mo
Now nature bit to N71102.09 g/mol
OCTU02
• moles of Salisylic acid = 3.00 x 10-3 mol 7
•theoretical yield
=
(0.002995 mois) (0.0105 mois
(3.00 × 1073) (0.0098) = 0.540 grams
10°
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 3 images

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax