Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter9: Atomic Absorption And Atomic Fluorescence Spectrometry
Section: Chapter Questions
Problem 9.6QAP
Related questions
Question
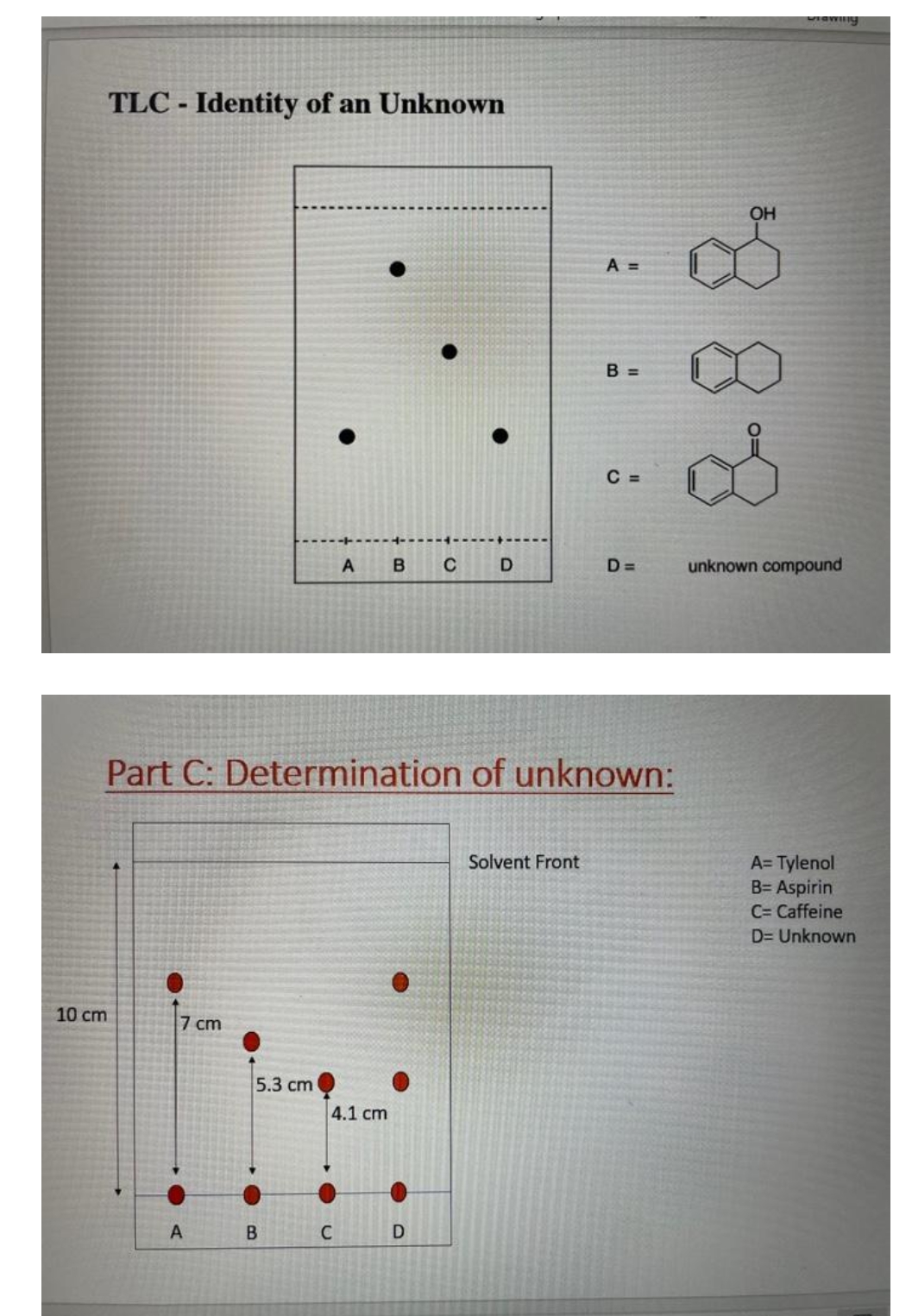
Transcribed Image Text:TLC - Identity of an Unknown
10 cm
7 cm
A
5.3 cm
B
A
Part C: Determination of unknown:
4.1 cm
C
B CD
A =
Solvent Front
B =
C =
OH
Drawing
D= unknown compound
A=Tylenol
B= Aspirin
C= Caffeine
D= Unknown
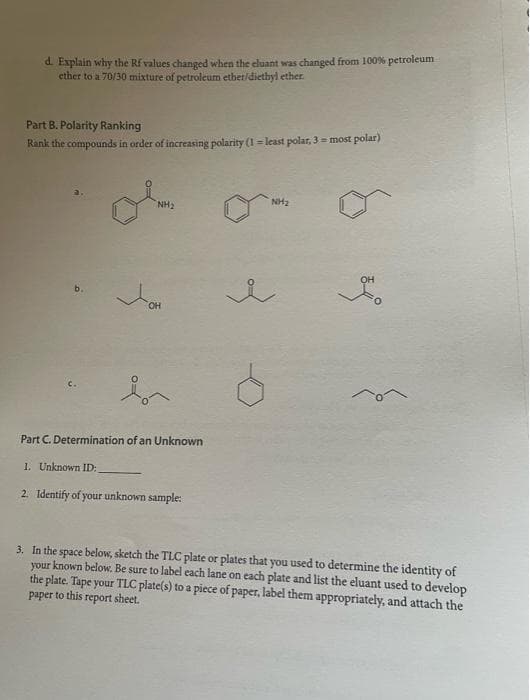
Transcribed Image Text:d. Explain why the Rf values changed when the cluant was changed from 100% petroleum
ether to a 70/30 mixture of petroleum ether/diethyl ether.
Part B. Polarity Ranking
Rank the compounds in order of increasing polarity (1= least polar, 3 = most polar)
NH₂
OH
Part C. Determination of an Unknown
1. Unknown ID:
2. Identify of your unknown sample:
NH₂
of
OH
3. In the space below, sketch the TLC plate or plates that you used to determine the identity of
your known below. Be sure to label each lane on each plate and list the eluant used to develop
the plate. Tape your TLC plate(s) to a piece of paper, label them appropriately, and attach the
paper to this report sheet.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
