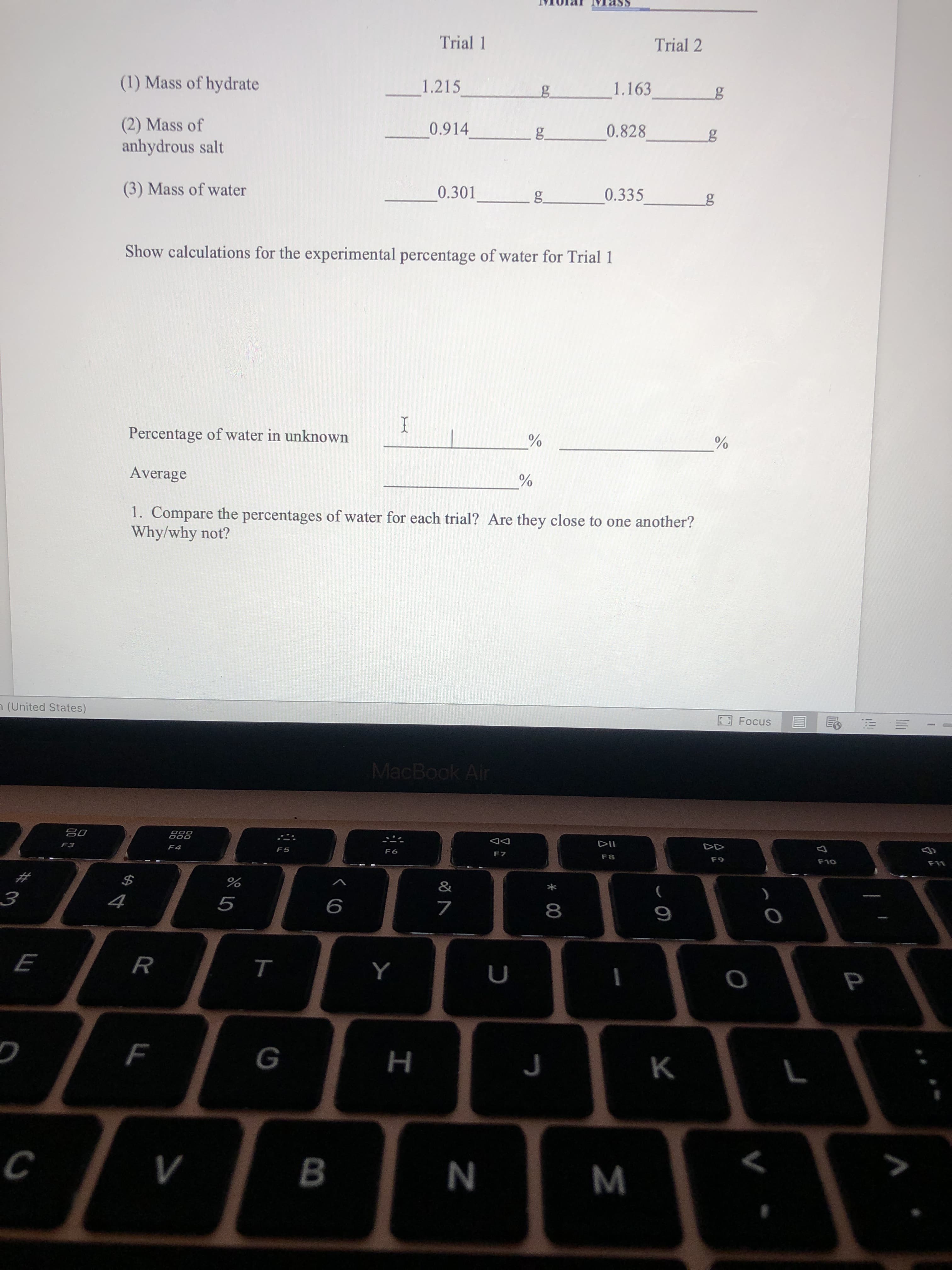
Trial 1 Trial 2 (1) Mass of hydrate 1.215 1.163 by (2) Mass of anhydrous salt 0.914 g. 0.828 (3) Mass of water 0.301 g. 0.335 Show calculations for the experimental percentage of water for Trial 1 Percentage of water in unknown I Average
Trial 1 Trial 2 (1) Mass of hydrate 1.215 1.163 by (2) Mass of anhydrous salt 0.914 g. 0.828 (3) Mass of water 0.301 g. 0.335 Show calculations for the experimental percentage of water for Trial 1 Percentage of water in unknown I Average
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.16QAP
Related questions
Question
Percentage of water and the average
Transcribed Image Text:* 00
T
Trial 1
Trial 2
(1) Mass of hydrate
1.215
1.163
0.828
(2) Mass of
anhydrous salt
0.914
(3) Mass of water
0.301
0.335
Show calculations for the experimental percentage of water for Trial 1
Percentage of water in unknown
Average
1. Compare the percentages of water for each trial? Are they close to one another?
Why/why not?
(United States)
snɔo
MacBook Air
000
DD
F5
F8
OL
&
%23
%
2$
5.
9
P:::
K.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

