EXERCISE Unit Conversion Drag conversion units onto the boxes in the equation to make conversions. Some boxes can be left empty. Click on a unit to remove it from its position. 1 cm Cu 9 g Cu 9.5 x 1021 1 g Cu atoms Cu 1 kg 1000 g 1 cm 1 mL 1L 1000 cm3 amount-840,units=kg,targetUnits=L amount=80,units-g,targetUnits-atoms amount=480000000,units=atoms,targetUnits=L A piece of copper has a mass of 840 kg. What is the volume of the sample, in units of liters? In the boxes above, enter the correct setup that would be used to solve this problem.
EXERCISE Unit Conversion Drag conversion units onto the boxes in the equation to make conversions. Some boxes can be left empty. Click on a unit to remove it from its position. 1 cm Cu 9 g Cu 9.5 x 1021 1 g Cu atoms Cu 1 kg 1000 g 1 cm 1 mL 1L 1000 cm3 amount-840,units=kg,targetUnits=L amount=80,units-g,targetUnits-atoms amount=480000000,units=atoms,targetUnits=L A piece of copper has a mass of 840 kg. What is the volume of the sample, in units of liters? In the boxes above, enter the correct setup that would be used to solve this problem.
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter3: Calculations With Chemical Formulas And Equaitons
Section3.4: Elemental Analysis: Percentages Of Carbon, Hydrogen And Oxygen
Problem 3.2CC
Related questions
Question
Could you help solve this ?
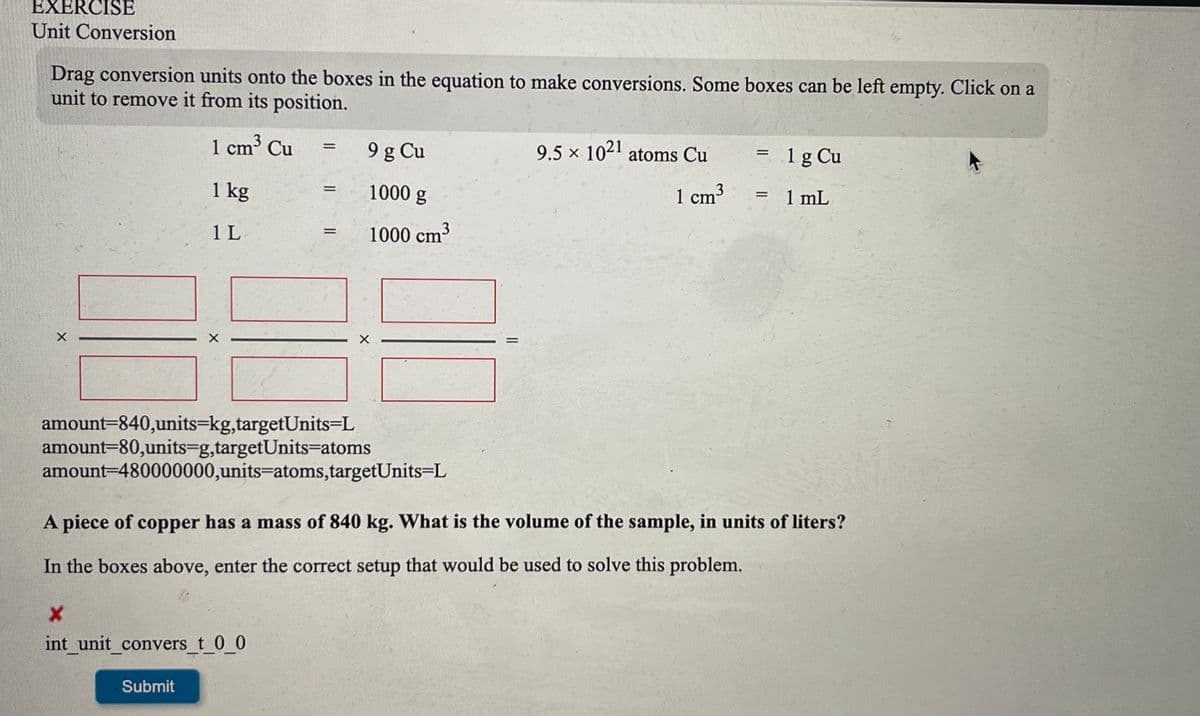
Transcribed Image Text:EXERCISE
Unit Conversion
Drag conversion units onto the boxes in the equation to make conversions. Some boxes can be left empty. Click on a
unit to remove it from its position.
1 cm³ Cu
9 g Cu
9.5 x 1021 atoms Cu
1g Cu
%D
1 kg
1000 g
1 cm3
1 mL
1 L
1000 cm³
amount=840,units=kg,targetUnits=L
amount=80,units=g,targetUnits=atoms
amount=480000000,units=atoms,targetUnits=L
A piece of copper has a mass of 840 kg. What is the volume of the sample, in units of liters?
In the boxes above, enter the correct setup that would be used to solve this problem.
int unit convers_t 0_0
Submit
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning