tudent needs 25.0 mL of 9.60x10 e No. 6 stock solution to work with, how much of the 2.00 mM solution should be added to the 25.0 mL volumetric sk? Hint, 2.00 mM is 0.00200 M. ) 8.330 mL -4 M FD&C Yellow Dye No. 6 solution. If the student has 2.00 mM FD&C Yellow 12.00 mL O 2.083 mL 0.08330 mL 10.50 mL ick Save and Submit to save and submit. Click Save All Answers to save all answers. Save All Answe
tudent needs 25.0 mL of 9.60x10 e No. 6 stock solution to work with, how much of the 2.00 mM solution should be added to the 25.0 mL volumetric sk? Hint, 2.00 mM is 0.00200 M. ) 8.330 mL -4 M FD&C Yellow Dye No. 6 solution. If the student has 2.00 mM FD&C Yellow 12.00 mL O 2.083 mL 0.08330 mL 10.50 mL ick Save and Submit to save and submit. Click Save All Answers to save all answers. Save All Answe
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.22QAP
Related questions
Question
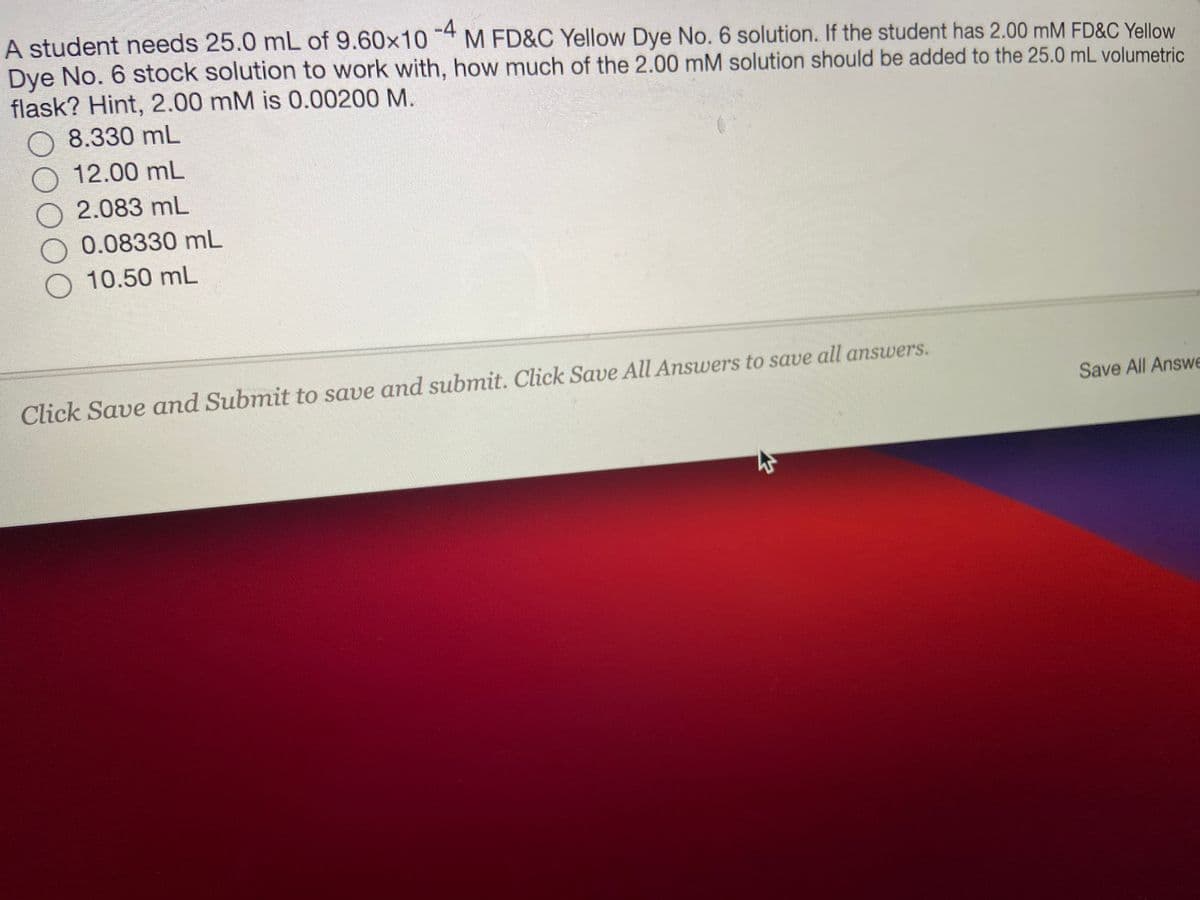
Transcribed Image Text:A student needs 25.0 mL of 9.60x10 4 M FD&C Yellow Dye No. 6 solution. If the student has 2.00 mM FD&C Yellow
Dye No. 6 stock solution to work with, how much of the 2.00 mM solution should be added to the 25.0 mL volumetric
flask? Hint, 2.00 mM is 0.00200 M.
8.330 mL
12.00 mL
2.083 mL
0.08330 mL
O 10.50 mL
Click Save and Submit to save and submit. Click Save All Answers to save all answers.
Save All Answe
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you



