Analytical Chemistry Laboratory for Biology Majors Data Sheet After the addition of increments of 0.50 mL NaOH to the Erlemeyer flask until the endpoint was reached, the volume contained was measured to be 47.037 ml Required: Volume (ml.) of NaOH Solution Delivered 47.037 ml - 33.187 mL = 13.85 mL After adding 25.0mL HCl and 0.50 mL phenolphthalein in a new 250.0 mL erlenmeyer flask, the volume contained was initially measured to be 25.5 ml. After the addition of increments of 0.001 mL NAOH to the Erlemeyer flask until the endpoint was reached, the volume contained was measured to be 27.685 ml. Required: Volume (ml) of NaOH Solution Delivered 27.685 ml - 25.500 ml. = 2.185 ml Required: Moles of HCI 1.06 Mx 0.002185 L = 2.3161x10 mol HCi Required: Molarity of HCI Solution Given: Moles HCl reacted with NaOH = 2. 3161x10³moles Volume of HCl = 25. 00 ml. x- moles HCI olume of HCI (L) 1000ml -= 0.02500 L Solution: M =- 23161r10 mol NCI 0.02500L NCI M = 0.09264 M
Analytical Chemistry Laboratory for Biology Majors Data Sheet After the addition of increments of 0.50 mL NaOH to the Erlemeyer flask until the endpoint was reached, the volume contained was measured to be 47.037 ml Required: Volume (ml.) of NaOH Solution Delivered 47.037 ml - 33.187 mL = 13.85 mL After adding 25.0mL HCl and 0.50 mL phenolphthalein in a new 250.0 mL erlenmeyer flask, the volume contained was initially measured to be 25.5 ml. After the addition of increments of 0.001 mL NAOH to the Erlemeyer flask until the endpoint was reached, the volume contained was measured to be 27.685 ml. Required: Volume (ml) of NaOH Solution Delivered 27.685 ml - 25.500 ml. = 2.185 ml Required: Moles of HCI 1.06 Mx 0.002185 L = 2.3161x10 mol HCi Required: Molarity of HCI Solution Given: Moles HCl reacted with NaOH = 2. 3161x10³moles Volume of HCl = 25. 00 ml. x- moles HCI olume of HCI (L) 1000ml -= 0.02500 L Solution: M =- 23161r10 mol NCI 0.02500L NCI M = 0.09264 M
Chapter11: Properties Of Solutions
Section: Chapter Questions
Problem 109AE: Patients undergoing an upper gastrointestinal tract laboratory test are typically given an X-ray...
Related questions
Question
According to this data why is HCl more concentrated than NaOH or vise versa?(correct the data if wrong)
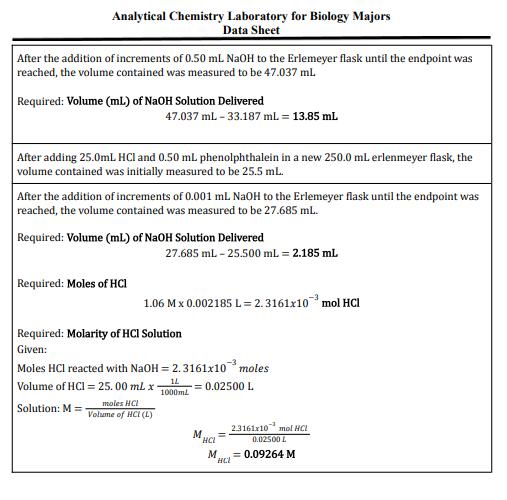
Transcribed Image Text:Analytical Chemistry Laboratory for Biology Majors
Data Sheet
After the addition of increments of 0.50 mL NAOH to the Erlemeyer flask until the endpoint was
reached, the volume contained was measured to be 47.037 ml
Required: Volume (ml.) of NaOH Solution Delivered
47.037 ml - 33.187 mL = 13.85 ml
After adding 25.0mL HCI and 0.50 mL phenolphthalein in a new 250.0 mL erlenmeyer flask, the
volume contained was initially measured to be 25.5 mL.
After the addition of increments of 0.001 mL NaOH to the Erlemeyer flask until the endpoint was
reached, the volume contained was measured to be 27.685 ml.
Required: Volume (mL) of NaOH Solution Delivered
27.685 ml - 25.500 ml = 2.185 ml
Required: Moles of HCI
1.06 M x 0.002185 L = 2.3161x10 mol HCI
Required: Molarity of HCI Solution
Given:
Moles HCl reacted with NaOH = 2.3161x10 moles
Volume of HCI = 25. 00 ml x-
1L
1000ml
= 0.02500 L
Solution: M =
moles HCI
Volume of HCI (L)
2.3161x10 mol HCI
M
HCI
0.02500 L
= 0.09264 M
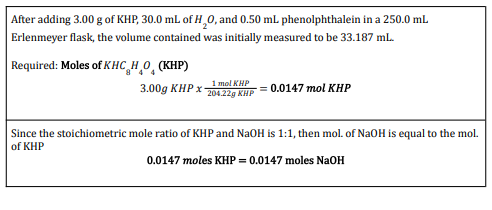
Transcribed Image Text:After adding 3.00 g of KHP, 30.0 ml of H,0, and 0.50 ml phenolphthalein in a 250.0 ml.
Erlenmeyer flask, the volume contained was initially measured to be 33.187 ml.
Required: Moles of KHC HO (KHP)
3.00g KHP x H = 0.0147 mol KHP
204.22g KHP
Since the stoichiometric mole ratio of KHP and NaOH is 1:1, then mol. of NaOH is equal to the mol.
of KHP
0.0147 moles KHP = 0.0147 moles NaOH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning
