Use the molecular orbital energy diagram below to answer the questions about bond order for the positive ion C, Number of Number of C Bond Order Bonding electrons Antibonding electrons This corresponds to: A. Single bond E. Between a single and double bond B. Double bond F. Between a double and a triple bond C. Triple bond G. No bond, C, does not form D. Half of a bond carbona MO's carbong 2p TL 2p - 2p 02p 414 PM FLV 9 Type here to search 4/23/2020 144 ins prt sc detete home end & + backspace iock T. home 08 LI hel , and drag to the Favorites Bar folder. Or import from another browser. Import favorites I2P 2p 02P T2p 25 2s 2s 02s o*1s 1s 1s O1s Try Another Version Submit Answer Progress: 0/1 item 4:14 PM 4/23/2020 FLV P Type here to search home ond prt sc ielete 144 pedseg→ Aock 8. %24 %23
Use the molecular orbital energy diagram below to answer the questions about bond order for the positive ion C, Number of Number of C Bond Order Bonding electrons Antibonding electrons This corresponds to: A. Single bond E. Between a single and double bond B. Double bond F. Between a double and a triple bond C. Triple bond G. No bond, C, does not form D. Half of a bond carbona MO's carbong 2p TL 2p - 2p 02p 414 PM FLV 9 Type here to search 4/23/2020 144 ins prt sc detete home end & + backspace iock T. home 08 LI hel , and drag to the Favorites Bar folder. Or import from another browser. Import favorites I2P 2p 02P T2p 25 2s 2s 02s o*1s 1s 1s O1s Try Another Version Submit Answer Progress: 0/1 item 4:14 PM 4/23/2020 FLV P Type here to search home ond prt sc ielete 144 pedseg→ Aock 8. %24 %23
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter10: Molecular Structure And Bonding Theories
Section: Chapter Questions
Problem 10.101QE: The molecular orbital diagram of NO shown in Figure 10.47 also applies to OF. Draw the complete...
Related questions
Question
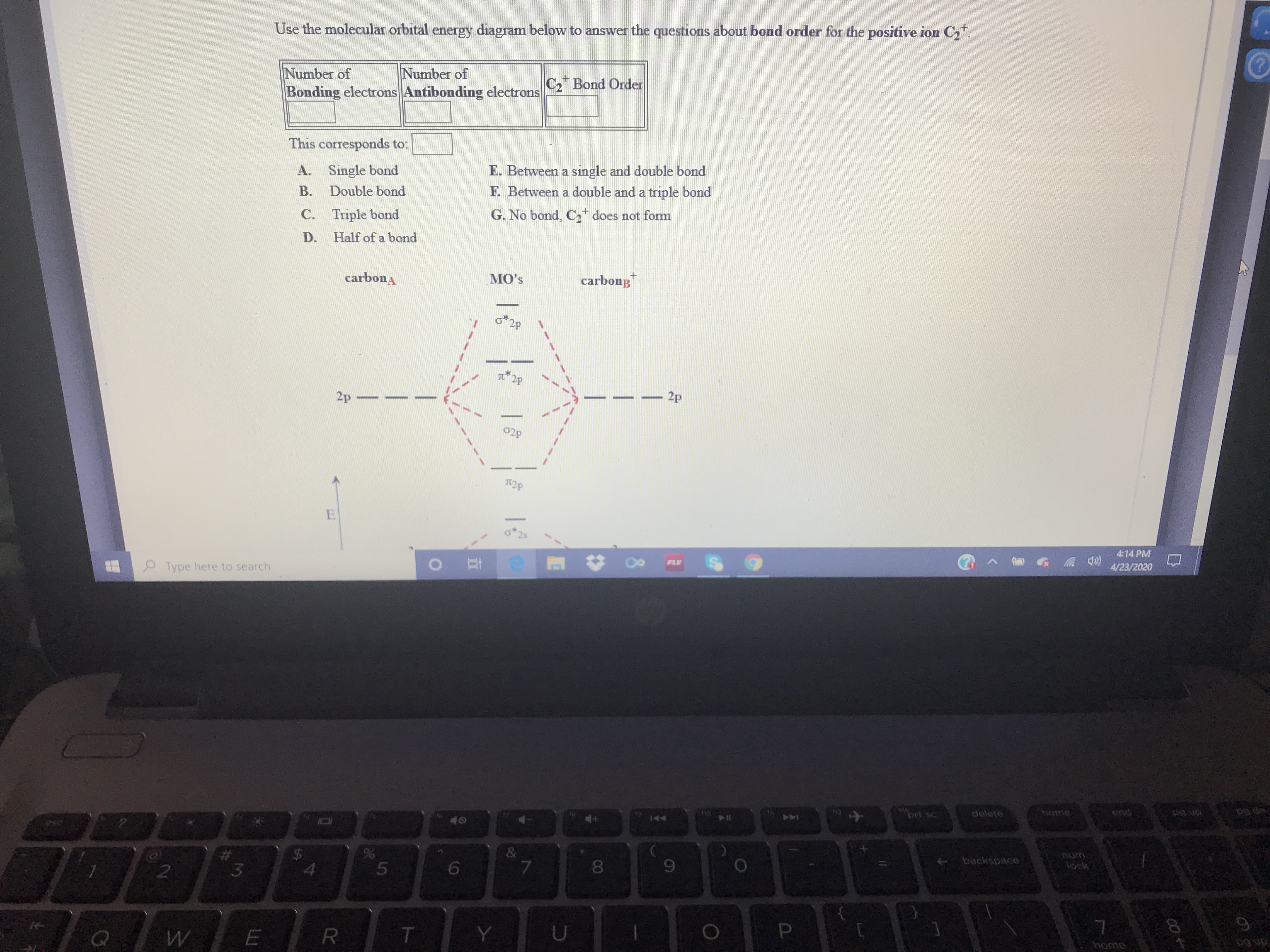
Transcribed Image Text:Use the molecular orbital energy diagram below to answer the questions about bond order for the positive ion C,
Number of
Number of
C Bond Order
Bonding electrons Antibonding electrons
This corresponds to:
A.
Single bond
E. Between a single and double bond
B.
Double bond
F. Between a double and a triple bond
C. Triple bond
G. No bond, C, does not form
D.
Half of a bond
carbona
MO's
carbong
2p
TL 2p
- 2p
02p
414 PM
FLV
9 Type here to search
4/23/2020
144
ins
prt sc
detete
home
end
&
+ backspace
iock
T.
home
08
LI
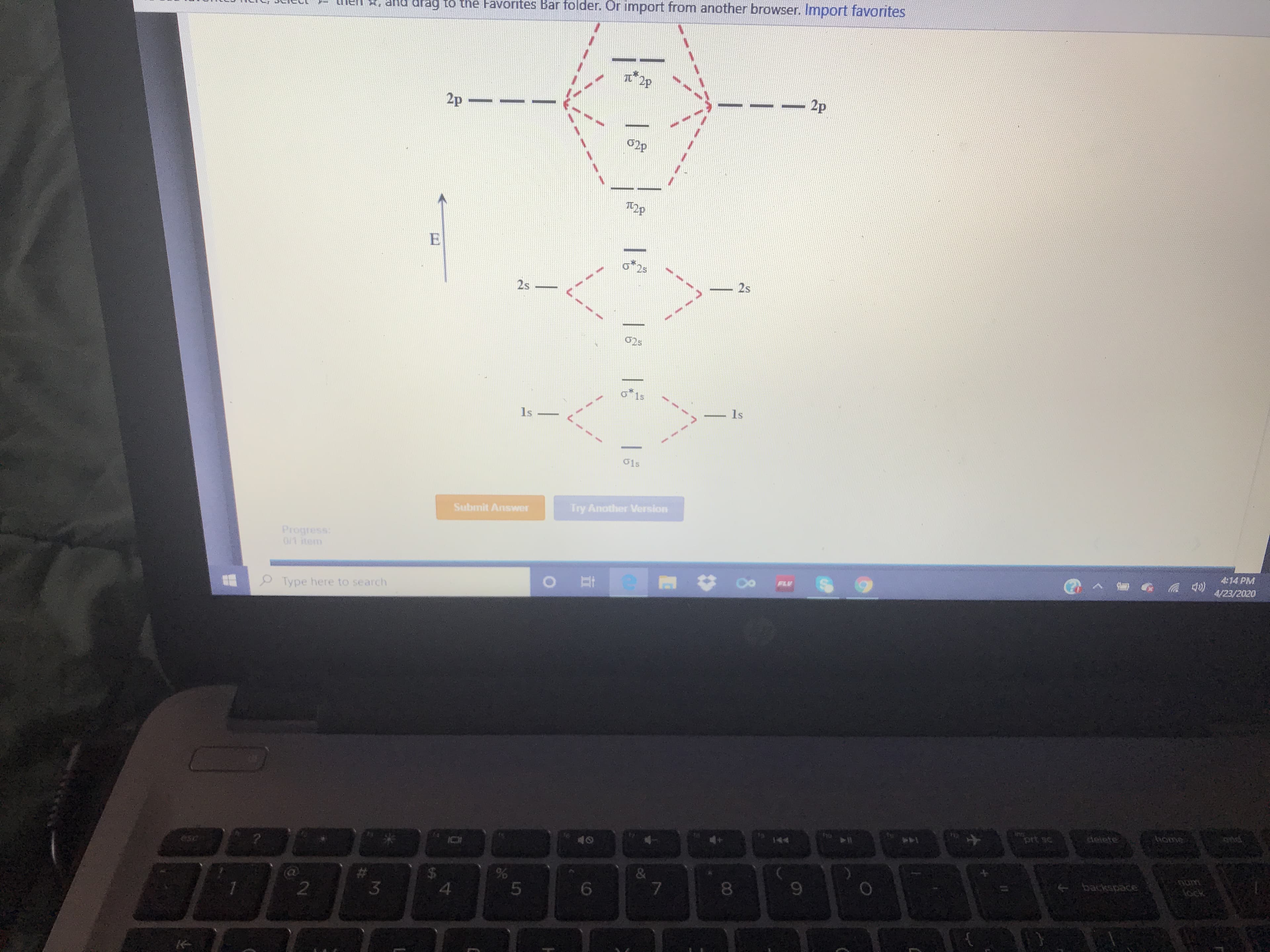
Transcribed Image Text:hel , and drag to the Favorites Bar folder. Or import from another browser. Import favorites
I2P
2p
02P
T2p
25
2s
2s
02s
o*1s
1s
1s
O1s
Try Another Version
Submit Answer
Progress:
0/1 item
4:14 PM
4/23/2020
FLV
P Type here to search
home
ond
prt sc
ielete
144
pedseg→
Aock
8.
%24
%23
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax