Use the References to access important values if needed for this question. In the laboratory, a general chemistry student measured the pH of a 0.580 M aqueous solution of codeine, C18 H21 O3 N to be 10.871. Use the information she obtained to determine the K₁ for this base. K(experiment) =| Submit Answer Retry Entire Group 9 more group attempts remaining
Use the References to access important values if needed for this question. In the laboratory, a general chemistry student measured the pH of a 0.580 M aqueous solution of codeine, C18 H21 O3 N to be 10.871. Use the information she obtained to determine the K₁ for this base. K(experiment) =| Submit Answer Retry Entire Group 9 more group attempts remaining
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter15: Acids And Bases
Section: Chapter Questions
Problem 15.127QP: A solution contains 4.25g of ammonia per 250.0 mL of solution. Electrical conductivity measurements...
Related questions
Question
In the laboratory, a general chemistry student measured the pH of a 0.580 M aqueous solution of codeine, C18 H21 O3N to be 10.871. Use the information she obtained to determine the Kb for this base. Kb(experiment)=
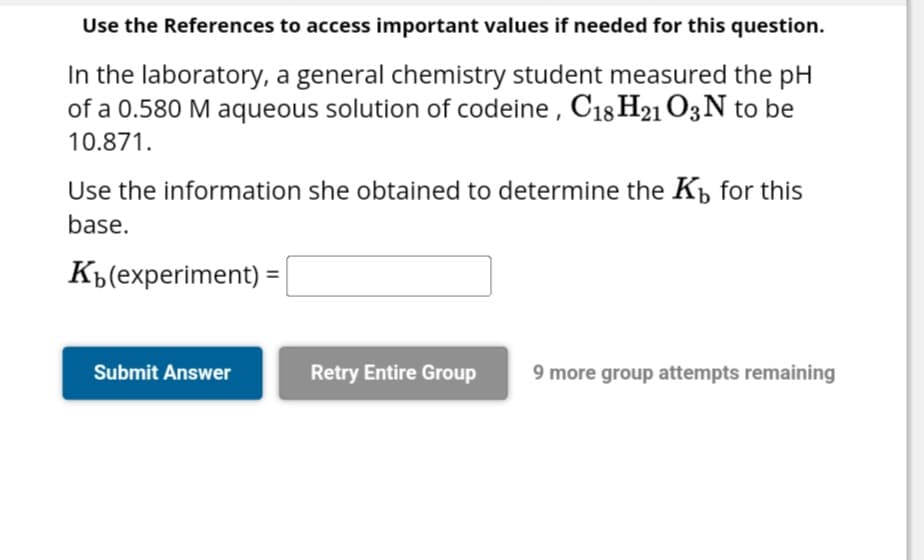
Transcribed Image Text:Use the References to access important values if needed for this question.
In the laboratory, a general chemistry student measured the pH
of a 0.580 M aqueous solution of codeine, C18 H21 O3 N to be
10.871.
Use the information she obtained to determine the K₁ for this
base.
K(experiment) =|
Submit Answer
Retry Entire Group
9 more group attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning