Use the References to access important values if needed for this question. The following information is given for diethyl ether at 1 atm: Ть 3 34.60°C ДНap (34.60°C) %3 357.5 Jg Tm =-116.30°C AHfus (-116.30°C) = 98.10 J/g Specific heat gas 1.460 J/g °C Specific heat liquid = 2.320 J/g °C A 46.50 g sample of liquid diethyl ether is initially at –1.30°C. If the sample is heated at constant pressure (P = 1 atm), kJ of energy are needed to raise the temperature of the sample to 55.00°C.
Use the References to access important values if needed for this question. The following information is given for diethyl ether at 1 atm: Ть 3 34.60°C ДНap (34.60°C) %3 357.5 Jg Tm =-116.30°C AHfus (-116.30°C) = 98.10 J/g Specific heat gas 1.460 J/g °C Specific heat liquid = 2.320 J/g °C A 46.50 g sample of liquid diethyl ether is initially at –1.30°C. If the sample is heated at constant pressure (P = 1 atm), kJ of energy are needed to raise the temperature of the sample to 55.00°C.
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter5: Thermochemistry
Section: Chapter Questions
Problem 5.97QE: The enthalpy of combustion of liquid n-hexane, C6H14, is 4159.5 kJ/mol, and that of gaseous n-hexane...
Related questions
Question
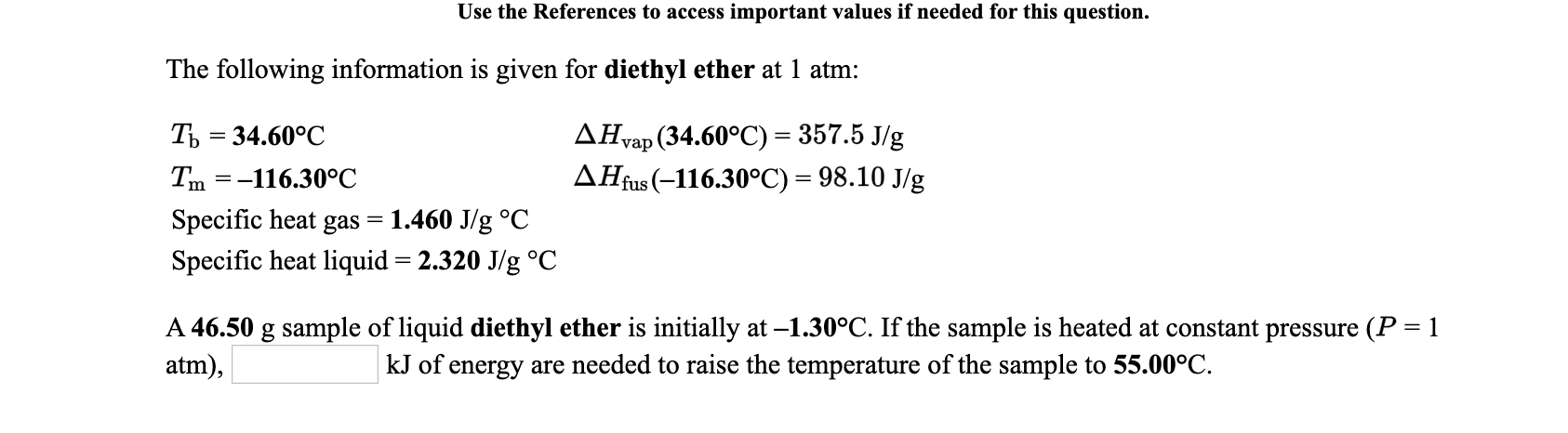
Transcribed Image Text:Use the References to access important values if needed for this question.
The following information is given for diethyl ether at 1 atm:
Ть 3 34.60°C
ДНap (34.60°C) %3 357.5 Jg
Tm =-116.30°C
AHfus (-116.30°C) = 98.10 J/g
Specific heat gas
1.460 J/g °C
Specific heat liquid = 2.320 J/g °C
A 46.50 g sample of liquid diethyl ether is initially at –1.30°C. If the sample is heated at constant pressure (P = 1
atm),
kJ of
energy are needed to raise the temperature of the sample to 55.00°C.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning