Chapter2: Basic Statistical Analysis With Excel
Section: Chapter Questions
Problem 12P
Related questions
Question
Use your average AA concentration to calculate the mass percent of acetic acid in vinegar.
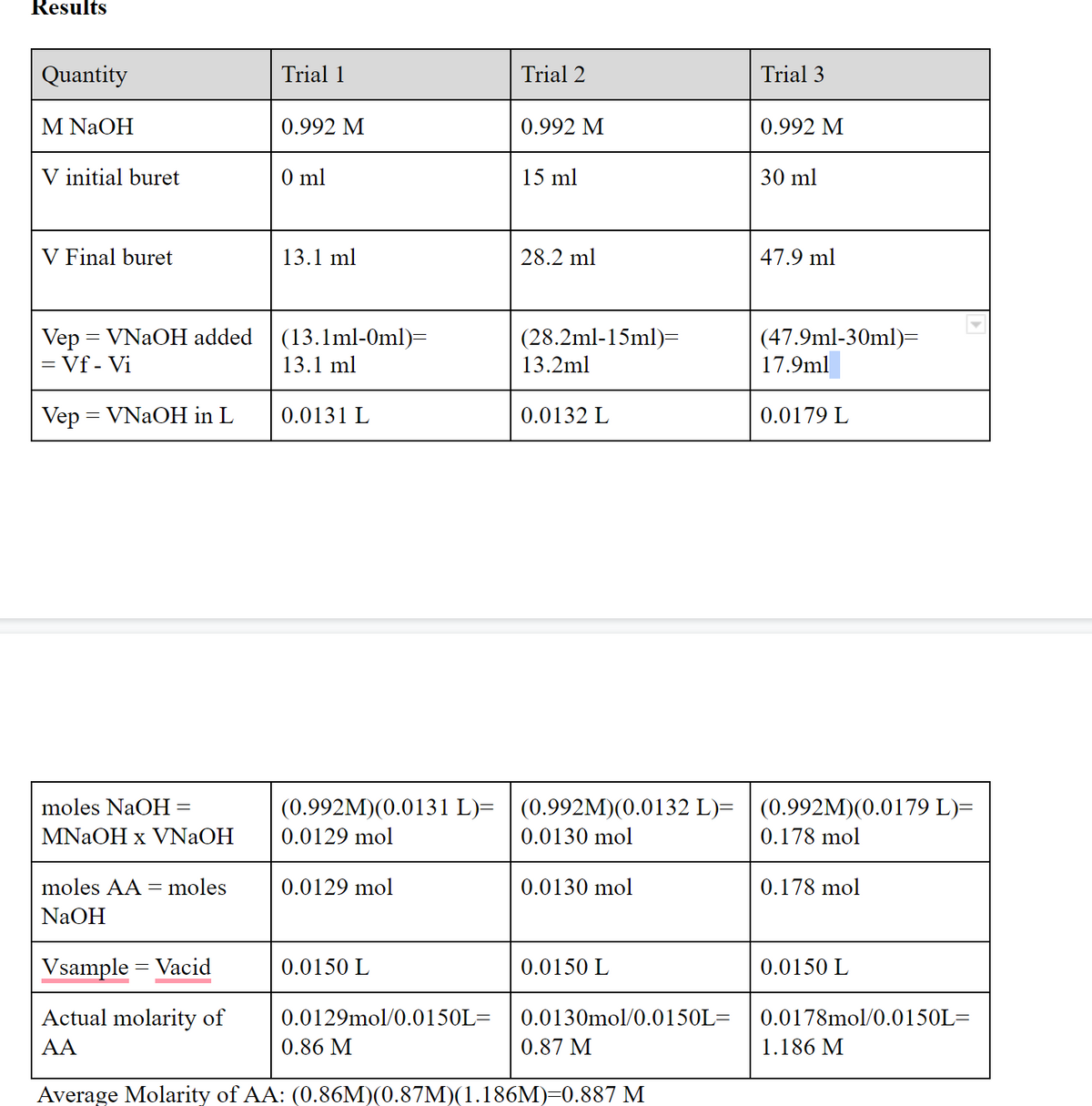
Transcribed Image Text:Results
Quantity
Trial 1
Trial 2
Trial 3
M NaOH
0.992 M
0.992 M
0.992 M
V initial buret
0 ml
15 ml
30 ml
V Final buret
13.1 ml
28.2 ml
47.9 ml
Vep = VNAOH added
= Vf - Vi
(47.9ml-30ml)=
(13.1ml-0ml)=
13.1 ml
(28.2ml-15ml)=
13.2ml
17.9ml
Vep = VNAOH in L
0.0131 L
0.0132 L
0.0179 L
moles NaOH=
(0.992M)(0.0131 L)= | (0.992M)(0.0132 L)= | (0.992M)(0.0179 L)=
MNAOH x VNaOH
0.0129 mol
0.0130 mol
0.178 mol
moles AA = moles
0.0129 mol
0.0130 mol
0.178 mol
NaOH
Vsample = Vacid
0.0150 L
0.0150 L
0.0150 L
Actual molarity of
0.0129mol/0.0150L=
0.0130mol/0.0150L=
0.0178mol/0.0150L=
AA
0.86 M
0.87 M
1.186 M
Average Molarity of AA: (0.86M)(0.87M)(1.186M)=0.887 M
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



