Use your calculated density of your unknown metal sphere to determine the mass of an object made from the same material that had a volume of 22.5 mL. Show the equation you use as well as your calculations and circle your final answer. Include units and the correct number of significant figures in your work and answer. A7. Use your calculated density of your unknown sphere to determine the volume of an object made from the same material that had a mass of 1.75 kg. Show the equation you use as well as your calculations and circle your final answer. Include units and the correct number of significant figures in your work and answer.
Use your calculated density of your unknown metal sphere to determine the mass of an object made from the same material that had a volume of 22.5 mL. Show the equation you use as well as your calculations and circle your final answer. Include units and the correct number of significant figures in your work and answer. A7. Use your calculated density of your unknown sphere to determine the volume of an object made from the same material that had a mass of 1.75 kg. Show the equation you use as well as your calculations and circle your final answer. Include units and the correct number of significant figures in your work and answer.
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter3: Measurement And Chemical Calculations
Section: Chapter Questions
Problem 128E: In Active Example 3-29 you calculated that you would have to work six weeks to earn enough money to...
Related questions
Question
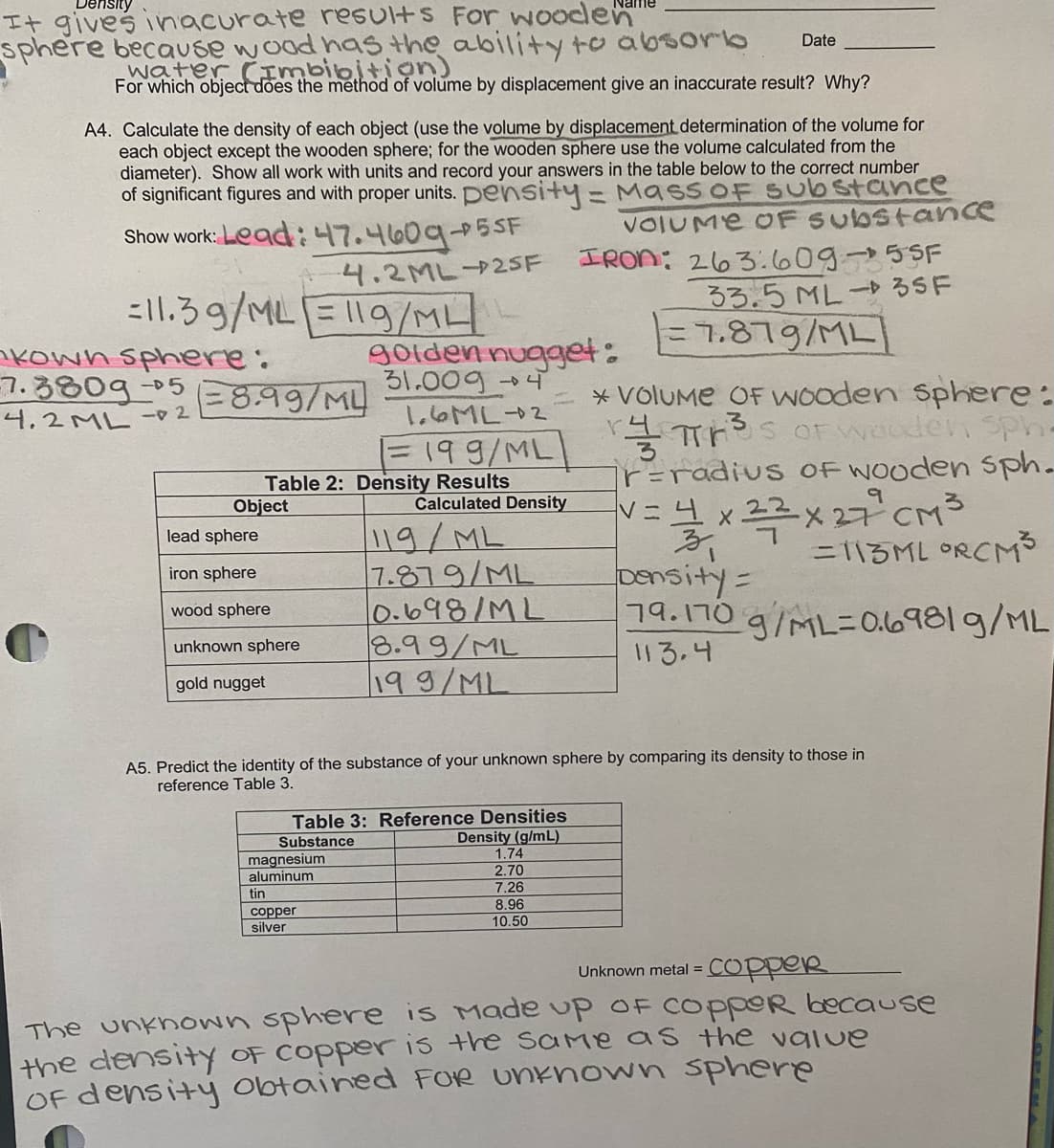
Transcribed Image Text:It gives inacurate results For Wooden"
sphere because wood haS the ability to absoro
water (imbibition)
Date
For which object does the method of volume by displacement give an inaccurate result? Why?
A4. Calculate the density of each object (use the volume by displacement determination of the volume for
each object except the wooden sphere; for the wooden sphere use the volume calculated from the
diameter). Show all work with units and record your answers in the table below to the correct number
of significant figures and with proper units. pensitty=MassOF Substance
VOIUME oF Substance
Show work: Lead: 47.460905.
5SF
IRON: 263:60955F
ろろ。5 ML-るSF
-니.2ML→25F
=11.39/MLE119/ML
%3D7.879/ML
nkown sphere:
7.380g-05
4.2ML 2
golden nugget:
31.0094
*VolUMe OF WOoden Sphere:
TS OFWOoden sph-
ir=radius OF WOoden Sph.
8.99/ML
1.6ML2
%3D199/ML|
Table 2: Density Results
Object
xx 27 CM3
bensity=
그9.170 g/ML=Ol0981 g/ML
Calculated Density
119/ML
7.879/ML
0.698/ML
3.99/ML
199/ML
lead sphere
=113ML ORCM3
iron sphere
wood sphere
unknown sphere
113.4
gold nugget
A5. Predict the identity of the substance of your unknown sphere by comparing its density to those in
reference Table 3.
Table 3: Reference Densities
Density (g/mL)
1.74
Substance
magnesium
aluminum
2.70
7.26
8.96
tin
соpper
silver
10.50
coppeR
Unknown metal =
The unkhown sphere is Made up OF COPPOR because
the density OF COPper is the same as the value
OF density obtained FOR UNknown sphere

Transcribed Image Text:A6. Use your calculated density of your unknown metal sphere to determine the mass of an object made
from the same material that had a volume of 22.5 mL. Show the equation you use as well as your
calculations and circle your final answer. Include units and the correct number of significant figures in
your work and answer.
A7. Use your calculated density of your unknown sphere to determine the volume of an object made
from the same material that had a mass of 1.75 kg. Show the equation you use as well as your
calculations and circle your final answer. Include units and the correct number of significant figures in
your work and answer.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning



Expert Answers to Latest Homework Questions
Q: H4/
Numerical Integration.
W
sin(x)
Q: As the real interest rate falls:
a) the supply of loanable funds increases.
b) the quantity supplied…
Q: What is the net gain (+) or loss (-) of the large country due to the tariff (compared to free…
Q: Proponents of the fixed exchange rate system argue that
a)flexible exchange rates may promote…
Q: 10) The table below shows the soft drink preferences of people in three age groups.
cola
root beer…
Q: A series of four annual constant-dollar payments beginning with $10,000 at the end of the first year…
Q: don't use excel only formula based typing answer.
Q: Do the Activity
Directions: Look and study the pictures of dessert below and write its
name,…
Q: Propose a synthesis for the following reaction
Need answer step by step
Q: Give proper exact answer to every part step by step and take a like
Q: Scenario: The Best Events Company organizes events, and business is booming, so much so that they…
Q: 8) A manufacturing process has a 70% yield, meaning that 70% of the products are acceptable
and 30%…
Q: Suppose a man purchases a luxury car with an initial down payment of $20,000 and then makes…
Q: Analyse the frame as shown in fig. 3 below by using Moment distribution method. And
Draw BMD and…
Q: Describe how the graph of the following function below transforms from the graph
of y = f(x).
2+
(0,…
Q: Image uploaded solution is not allowed please dear expert
Q: Image uploaded solution is not allowed please dear expert.
Q: Merchant's ship is sailing on the equator. During the sail, a container falls from the ship to the…
Q: At a large round table sit n ≥ 2 players, each holding 3 cards: one white, one black, and one red.…
Q: 20kN
B
C
SITUATION 2: A plane truss is loaded as shown. Determine the:
4. force in member BC.
5.…
Q: A 11 m tall streetlight is positioned 16 m away from a vertical wall.
Timothy throws a tennis ball…