V. P. Kolesov et al. (J. Chem Thermodynamics 28 (1996) 1121) reported the standard enthalpy or combustion AH" and the standard enthalpy of formation 4H of crystalline C6o based on bomb calorimeter measurements. In one of their runs they found the standard specific internal energy of combustion of C60 to be – 36.0334 kJ g'at 298.15K and 1 bar pressure. Calculate 4,H° and 4H° for C60 at 298.15K and 1 bar pressure. Data in the following table may be helpful. Specific refers to heat per gram. Substance CO2(g) H2O() 1. AH in kJ mol at at 298.15K and 1 bar -393.51 -285.83
V. P. Kolesov et al. (J. Chem Thermodynamics 28 (1996) 1121) reported the standard enthalpy or combustion AH" and the standard enthalpy of formation 4H of crystalline C6o based on bomb calorimeter measurements. In one of their runs they found the standard specific internal energy of combustion of C60 to be – 36.0334 kJ g'at 298.15K and 1 bar pressure. Calculate 4,H° and 4H° for C60 at 298.15K and 1 bar pressure. Data in the following table may be helpful. Specific refers to heat per gram. Substance CO2(g) H2O() 1. AH in kJ mol at at 298.15K and 1 bar -393.51 -285.83
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
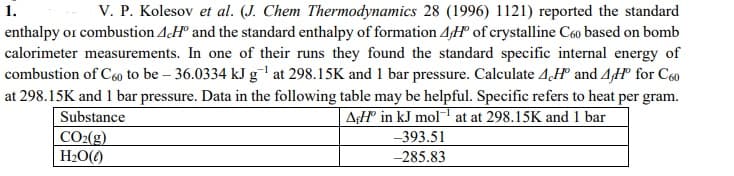
Transcribed Image Text:V. P. Kolesov et al. (J. Chem Thermodynamics 28 (1996) 1121) reported the standard
enthalpy or combustion AH° and the standard enthalpy of formation 4H° of crystalline C60 based on bomb
calorimeter measurements. In one of their runs they found the standard specific internal energy of
combustion of C60 to be – 36.0334 kJ g' at 298.15K and 1 bar pressure. Calculate 4,H° and 4H° for C60
1.
at 298.15K and 1 bar pressure. Data in the following table may be helpful. Specific refers to heat per gram.
Substance
A¢H in kJ mol at at 298.15K and 1 bar
CO2(g)
H2O()
-393.51
-285.83
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY