vinegar solution, and mass % of acetic acid in the vinegar used to titrate 5.00 mL of the vinegar were calculated using the average of three good trials (difference no greater than 0.1 mL). Data&Observations Volume of NAOH used in the titration: Rough trial Trial Trial 2 Trial 3 Initial reading (mL) Final reading (mL) Volume 0.00 0.00 0.00 0.00 25.50 25.90 25.80 26.00 25.50 25.90 25.80 26.00 dispensed (mL) Average volume of NaOH used (Trials 1-3) 25.90 mL Molarity of NaOH = 0.200 M should ze shosn unda CalmeaOi Unknown Vinegar # 18 The actual molarity of the vinegar = 1.00 M The actual mass % of acetic acid 5.95 % A faint pink color was observed at the end point of each titration trial. Calculations& Results Calculations and results pages are attached. Below is a set of caleulation performed in this experiment: Calculation of moles HC,HaO, in 5.00 mL vinegar: L NaOHmol NaOH mol HC2H302 Average mL NaOH 0.200 mol NaOH 1 mol HC2H3 02) 1 L NaOH = 0.00518 mol HC2H302 1 L NaOH 1 mol NaOH 25.90 mL NAOH 1000 mL NAOH 5 Aion of molarity of vinegar: mol HC2H302 -molarity of vinegar 1 0.00518 mol HC2H302 = 1.04 M 0.00500 L vineg ar Calculation of mass % of acetic acid in 5.00 mL of vinegar: mass of acetic acid mass % acetic acid in vineg ar X 100 mass of vinegar 60.05 g HC2H3O2 mass of HC2H3 O2 0.00518 mol HC2H302 = = 0.311 g HC2H302 1 mol HC2H3O2 1.01 g vinegar mass of vinegar = 5.00 mL vineg ar = 5.05 g vineg ar 1.00 mL vineg ar 0.311 g HC2H3 O2 mass % acetic acid in vineg ar X 100 6.16 % 5.05 g vinegar The % error in the calculation of the molarity of the vinegar: lexperimental molarity - actual molarity actual molarity X 100 % error in mo larity of vinegar 1 1.04 M - 1.00 M| X 100 1.00 M = 4% The % error in the calculation of the mass % of acetic acid in vinegar: lexperimental mass %- actual mass % actual mass % x 100 % error in mass % of ace tic acid = 16.16%-5.95%| 5.95% - 100 = 3.5% io Ctu o Lib) elel boau HIOsVlo omu gail ein yaib
vinegar solution, and mass % of acetic acid in the vinegar used to titrate 5.00 mL of the vinegar were calculated using the average of three good trials (difference no greater than 0.1 mL). Data&Observations Volume of NAOH used in the titration: Rough trial Trial Trial 2 Trial 3 Initial reading (mL) Final reading (mL) Volume 0.00 0.00 0.00 0.00 25.50 25.90 25.80 26.00 25.50 25.90 25.80 26.00 dispensed (mL) Average volume of NaOH used (Trials 1-3) 25.90 mL Molarity of NaOH = 0.200 M should ze shosn unda CalmeaOi Unknown Vinegar # 18 The actual molarity of the vinegar = 1.00 M The actual mass % of acetic acid 5.95 % A faint pink color was observed at the end point of each titration trial. Calculations& Results Calculations and results pages are attached. Below is a set of caleulation performed in this experiment: Calculation of moles HC,HaO, in 5.00 mL vinegar: L NaOHmol NaOH mol HC2H302 Average mL NaOH 0.200 mol NaOH 1 mol HC2H3 02) 1 L NaOH = 0.00518 mol HC2H302 1 L NaOH 1 mol NaOH 25.90 mL NAOH 1000 mL NAOH 5 Aion of molarity of vinegar: mol HC2H302 -molarity of vinegar 1 0.00518 mol HC2H302 = 1.04 M 0.00500 L vineg ar Calculation of mass % of acetic acid in 5.00 mL of vinegar: mass of acetic acid mass % acetic acid in vineg ar X 100 mass of vinegar 60.05 g HC2H3O2 mass of HC2H3 O2 0.00518 mol HC2H302 = = 0.311 g HC2H302 1 mol HC2H3O2 1.01 g vinegar mass of vinegar = 5.00 mL vineg ar = 5.05 g vineg ar 1.00 mL vineg ar 0.311 g HC2H3 O2 mass % acetic acid in vineg ar X 100 6.16 % 5.05 g vinegar The % error in the calculation of the molarity of the vinegar: lexperimental molarity - actual molarity actual molarity X 100 % error in mo larity of vinegar 1 1.04 M - 1.00 M| X 100 1.00 M = 4% The % error in the calculation of the mass % of acetic acid in vinegar: lexperimental mass %- actual mass % actual mass % x 100 % error in mass % of ace tic acid = 16.16%-5.95%| 5.95% - 100 = 3.5% io Ctu o Lib) elel boau HIOsVlo omu gail ein yaib
Chapter31: Introduction To Analytical Separations
Section: Chapter Questions
Problem 31.19QAP
Related questions
Question
How did he find the actual Molarity (1.00M)of vinegar and actual mass % of acetic acid ?( 5.95%)
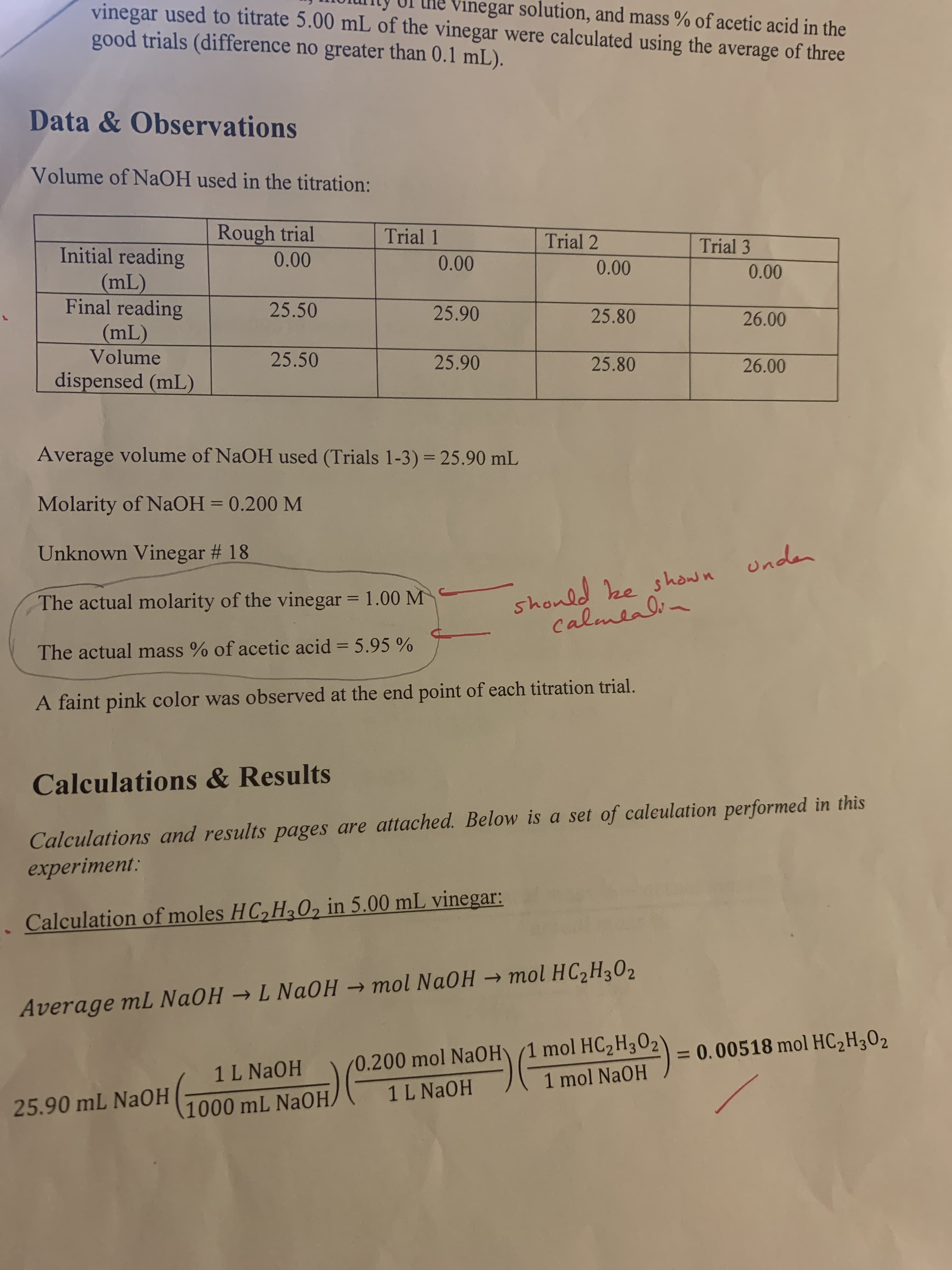
Transcribed Image Text:vinegar solution, and mass % of acetic acid in the
vinegar used to titrate 5.00 mL of the vinegar were calculated using the average of three
good trials (difference no greater than 0.1 mL).
Data&Observations
Volume of NAOH used in the titration:
Rough trial
Trial
Trial 2
Trial 3
Initial reading
(mL)
Final reading
(mL)
Volume
0.00
0.00
0.00
0.00
25.50
25.90
25.80
26.00
25.50
25.90
25.80
26.00
dispensed (mL)
Average volume of NaOH used (Trials 1-3) 25.90 mL
Molarity of NaOH = 0.200 M
should ze shosn unda
CalmeaOi
Unknown Vinegar # 18
The actual molarity of the vinegar = 1.00 M
The actual mass % of acetic acid 5.95 %
A faint pink color was observed at the end point of each titration trial.
Calculations& Results
Calculations and results pages are attached. Below is a set of caleulation performed in this
experiment:
Calculation of moles HC,HaO, in 5.00 mL vinegar:
L NaOHmol NaOH mol HC2H302
Average mL NaOH
0.200 mol NaOH 1 mol HC2H3 02)
1 L NaOH
= 0.00518 mol HC2H302
1 L NaOH
1 mol NaOH
25.90 mL NAOH
1000 mL NAOH

Transcribed Image Text:5
Aion of molarity of vinegar:
mol HC2H302
-molarity of vinegar
1
0.00518 mol HC2H302
= 1.04 M
0.00500 L vineg ar
Calculation of mass % of acetic acid in 5.00 mL of vinegar:
mass of acetic acid
mass % acetic acid in vineg ar
X 100
mass of vinegar
60.05 g HC2H3O2
mass of HC2H3 O2
0.00518 mol HC2H302
=
= 0.311 g HC2H302
1 mol HC2H3O2
1.01 g vinegar
mass of vinegar
= 5.00 mL vineg ar
= 5.05 g vineg ar
1.00 mL vineg ar
0.311 g HC2H3 O2
mass % acetic acid in vineg ar
X 100 6.16 %
5.05 g vinegar
The % error in the calculation of the molarity of the vinegar:
lexperimental molarity - actual molarity
actual molarity
X 100
% error in mo larity of vinegar
1
1.04 M - 1.00 M|
X 100
1.00 M
= 4%
The % error in the calculation of the mass % of acetic acid in vinegar:
lexperimental mass %- actual mass %
actual mass %
x 100
% error in mass % of ace tic acid =
16.16%-5.95%|
5.95%
- 100
= 3.5%
io
Ctu o
Lib) elel
boau HIOsVlo omu
gail ein
yaib
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning