What color was the indicator for acidic solutions? What about for basic and neutral solutions? What was the order of your samples, from most acidic to least acidic? (b) What is the organic compound responsible for the color changes observed? What is the basis for these color changes?
What color was the indicator for acidic solutions? What about for basic and neutral solutions? What was the order of your samples, from most acidic to least acidic? (b) What is the organic compound responsible for the color changes observed? What is the basis for these color changes?
Chapter15: The Chemistry Of Household Products
Section: Chapter Questions
Problem 4E
Related questions
Question
(a) What color was the indicator for acidic solutions? What about for basic and neutral solutions? What was the order of your samples, from most acidic to least acidic?
(b) What is the organic compound responsible for the color changes observed? What is the basis for these color changes?
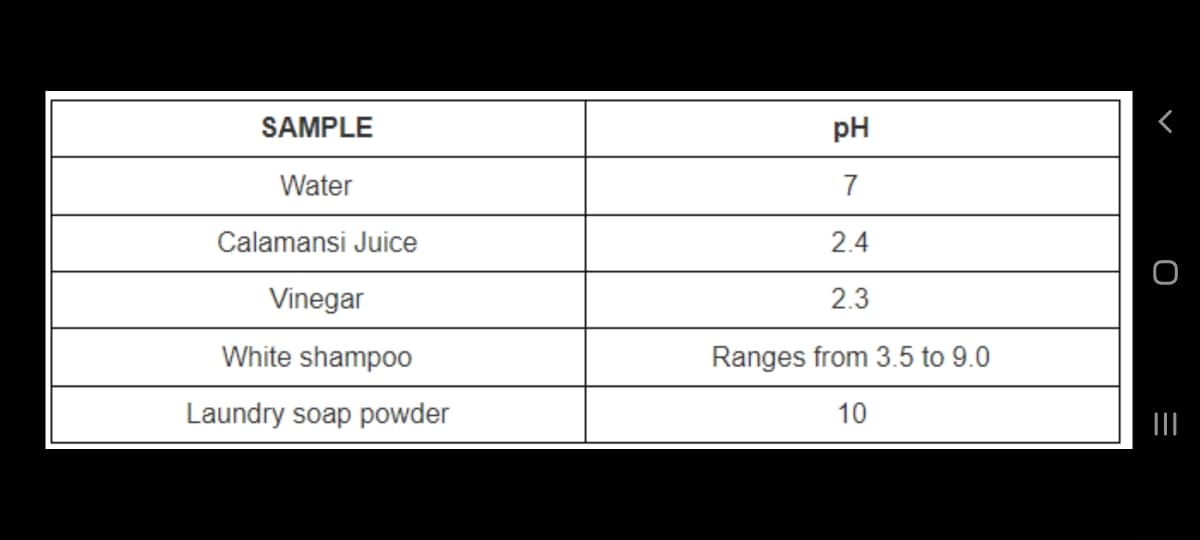
Transcribed Image Text:SAMPLE
pH
Water
7
Calamansi Juice
2.4
Vinegar
2.3
White shampoo
Ranges from 3.5 to 9.0
Laundry soap powder
10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning