Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter13: Substitution
Section: Chapter Questions
Problem 43CTQ
Related questions
Question
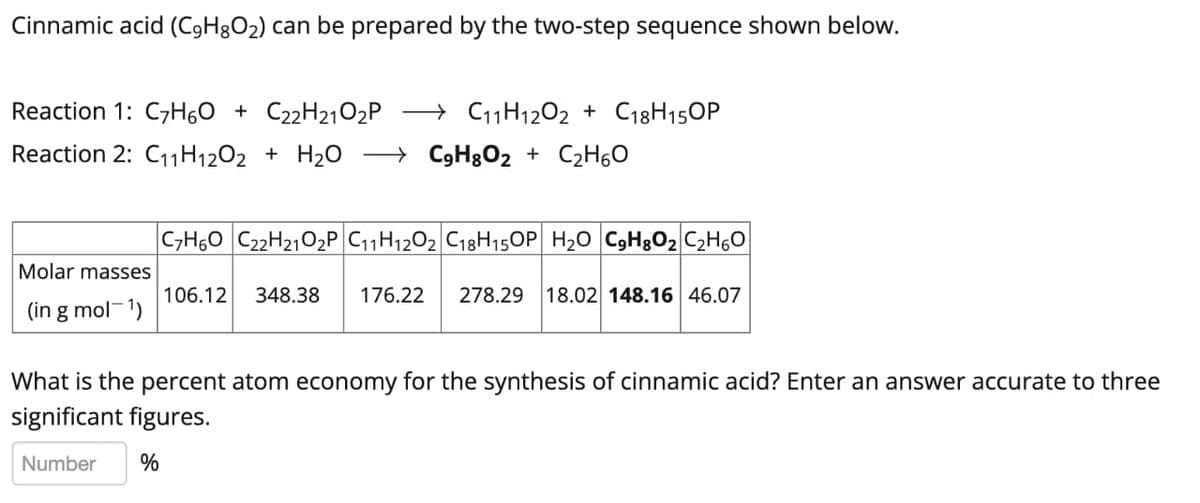
Transcribed Image Text:Cinnamic acid (C9H8O2) can be prepared by the two-step sequence shown below.
Reaction 1: C7H60 + C22H21O2P
→ C11H1202 + C18H150P
Reaction 2: C11H1202 + H2O → C9H3O2 + C2H60
|C,H60 C22H2102P C11H1202 C18H15OP H20 C9H3O2 C2H6O
Molar masses
106.12
348.38
176.22
278.29
18.02 148.16 46.07
(in g mol-1)
What is the percent atom economy for the synthesis of cinnamic acid? Enter an answer accurate to three
significant figures.
Number
%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning