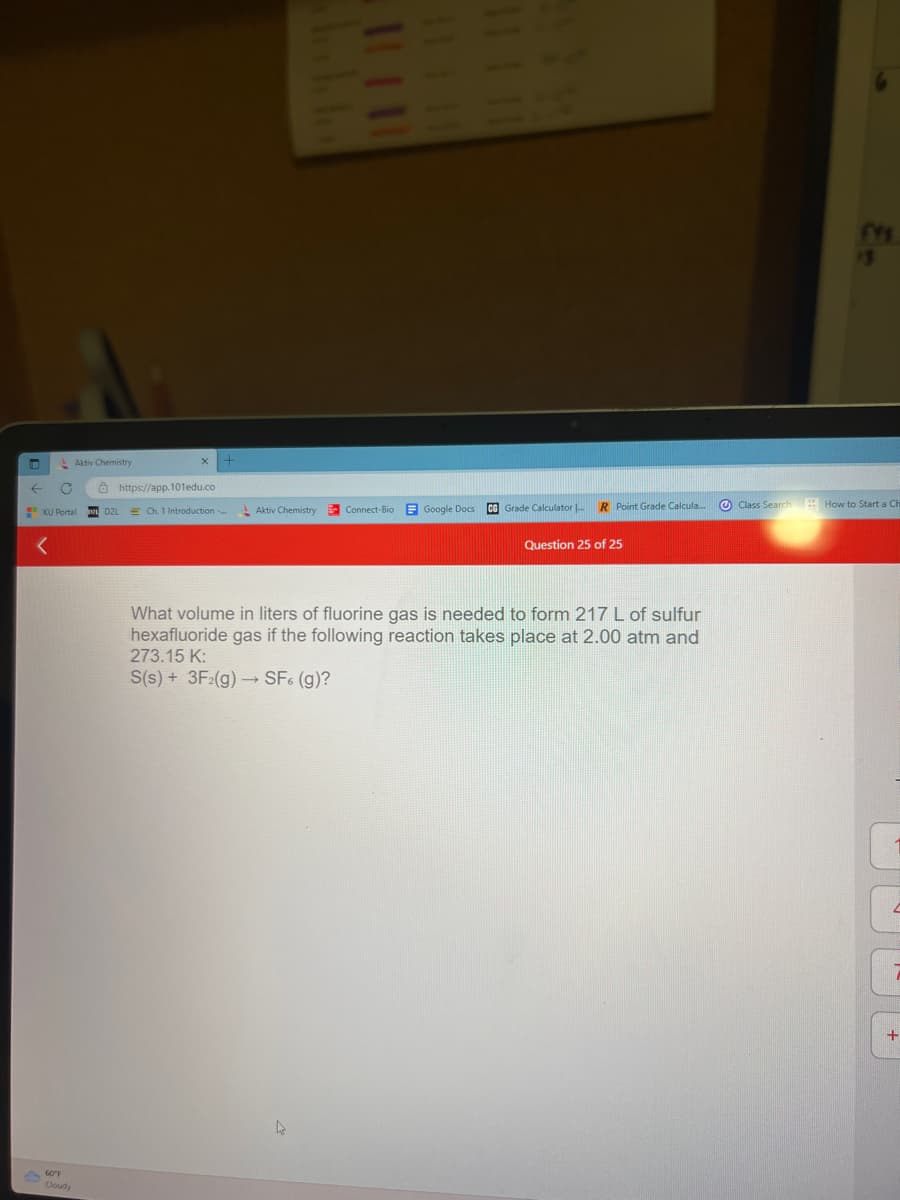
What volume in liters of fluorine gas is needed to form 217 L of sulfur hexafluoride gas if the following reaction takes place at 2.00 atm and 273.15 K: S(s) + 3F2(g) → SF6 (g)?
What volume in liters of fluorine gas is needed to form 217 L of sulfur hexafluoride gas if the following reaction takes place at 2.00 atm and 273.15 K: S(s) + 3F2(g) → SF6 (g)?
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter8: Properties Of Gases
Section: Chapter Questions
Problem 122QRT
Related questions
Question
Transcribed Image Text:T
← C
Aktiv Chemistry
60°F
Cloudy
KU Portal DRL D2L
X
https://app.101edu.co
+
E Ch. 1 Introduction...
Aktiv Chemistry
Connect-Bio
111 11
Google Docs
CG Grade Calculator ...
R Point Grade Calcula...
Question 25 of 25
What volume in liters of fluorine gas is needed to form 217 L of sulfur
hexafluoride gas if the following reaction takes place at 2.00 atm and
273.15 K:
S(s) + 3F2(g) → SF6 (g)?
CYS
Class Search How to Start a Ch
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning