When glucose, a sugar, reacts fully with oxygen, carbon dioxide and water are produced: C,H12O6(s) + 6 O2(g) → 6 CO2(g) + 6 H,O(€) AH° = -2820 kJ Suppose a person weighing 50 kg (mostly water, with spe- cific heat capacity 4.18 J K-1 g¯') eats a candy bar con- taining 14.3 g glucose. If all the glucose reacted with oxygen and the heat produced were used entirely to increase the person's body temperature, what temperature increase would result? (In fact, most of the heat produced is lost to the surroundings before such a temperature increase occurs.)
When glucose, a sugar, reacts fully with oxygen, carbon dioxide and water are produced: C,H12O6(s) + 6 O2(g) → 6 CO2(g) + 6 H,O(€) AH° = -2820 kJ Suppose a person weighing 50 kg (mostly water, with spe- cific heat capacity 4.18 J K-1 g¯') eats a candy bar con- taining 14.3 g glucose. If all the glucose reacted with oxygen and the heat produced were used entirely to increase the person's body temperature, what temperature increase would result? (In fact, most of the heat produced is lost to the surroundings before such a temperature increase occurs.)
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter12: Thermodynamic Processes And Thermochemistry
Section: Chapter Questions
Problem 71AP
Related questions
Question
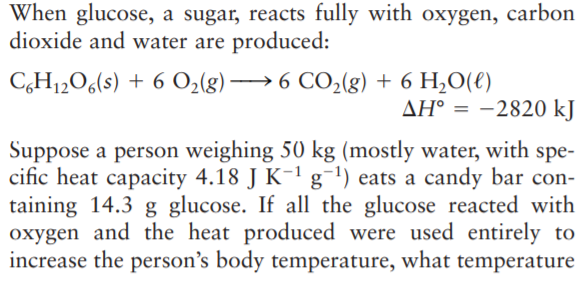
Transcribed Image Text:When glucose, a sugar, reacts fully with oxygen, carbon
dioxide and water are produced:
C,H12O6(s) + 6 O2(g) → 6 CO2(g) + 6 H,O(€)
AH° = -2820 kJ
Suppose a person weighing 50 kg (mostly water, with spe-
cific heat capacity 4.18 J K-1 g¯') eats a candy bar con-
taining 14.3 g glucose. If all the glucose reacted with
oxygen and the heat produced were used entirely to
increase the person's body temperature, what temperature

Transcribed Image Text:increase would result? (In fact, most of the heat produced
is lost to the surroundings before such a temperature
increase occurs.)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning