When the me pure benzoic acid or mandelic acid is reached: Hting point of T/F) Dipale-dipole forces are broken OF The temp. remains essentially constant TF Dispersian forces are broken. TI Covalent bonds are broken, TF H bonds are broken
When the me pure benzoic acid or mandelic acid is reached: Hting point of T/F) Dipale-dipole forces are broken OF The temp. remains essentially constant TF Dispersian forces are broken. TI Covalent bonds are broken, TF H bonds are broken
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter4: Polar Bonds, Polar Reactions
Section: Chapter Questions
Problem 2CTQ: Figure 4.1 is a cartoon depiction of liquid water at the molecular level. a. (E) Label at least one...
Related questions
Question
I got this question wrong. Can someone explain why? I thought dipole forces would not be broken no matter the temperature.
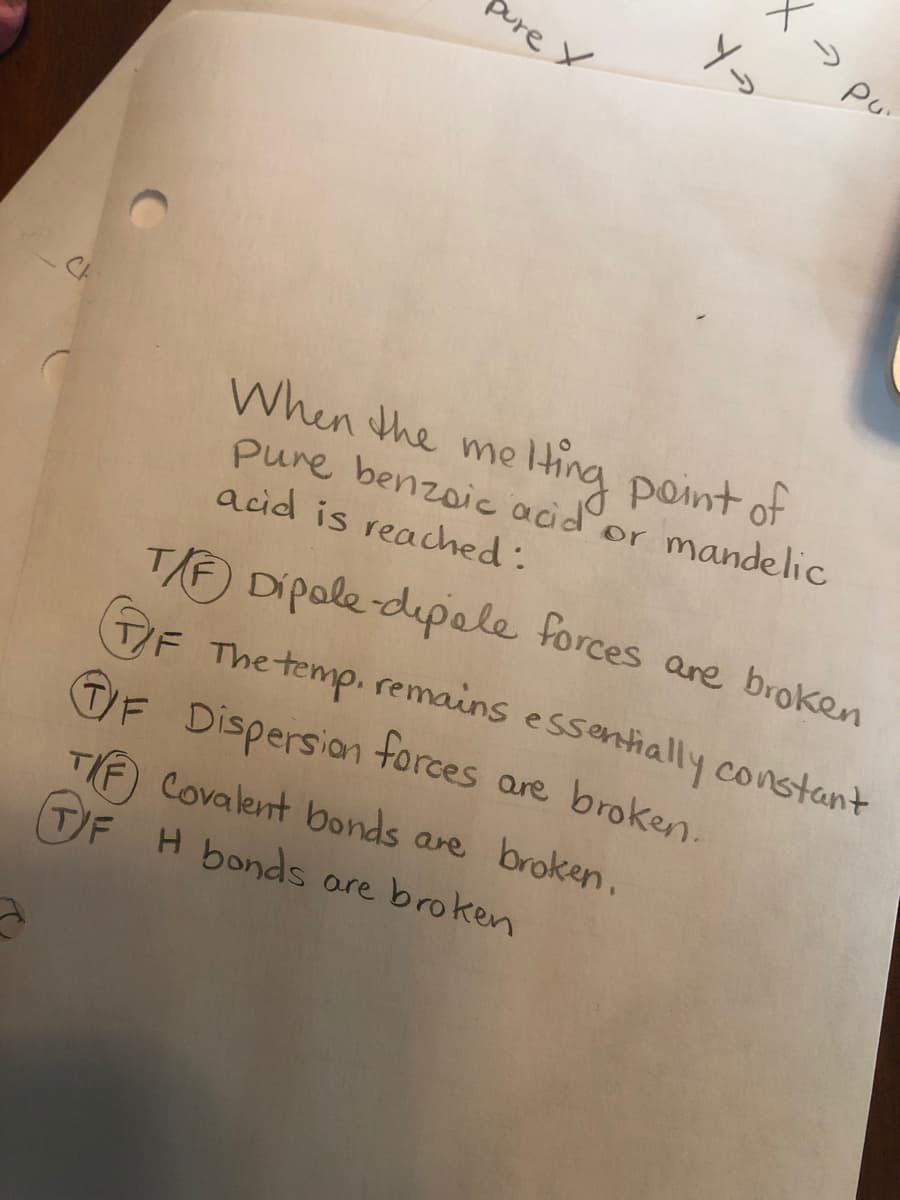
Transcribed Image Text:Sure X
When the me
pure benzoic acid or mandelic
acid is reached:
ting point of
T/F) Dipale-dipole forces are broken
T/F The temp. remains essentially constant
TF Dispersion forces are broken.
Covalent bonds are broken,
TIF
TF H bonds are broken
> pu"
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning