Which of the following molecular formulas is consistent with the mass spectrum shown below? A) C,H3O 100 - 80 B) C2H;Br 60- C) C2H4 20 D) C,HgN 10 20 30 40 50 70 80 90 100 110 m/z E) None of the above. Relative Intensity
Which of the following molecular formulas is consistent with the mass spectrum shown below? A) C,H3O 100 - 80 B) C2H;Br 60- C) C2H4 20 D) C,HgN 10 20 30 40 50 70 80 90 100 110 m/z E) None of the above. Relative Intensity
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter14: Mass Spectrometry
Section: Chapter Questions
Problem 14.28P: Following is the mass spectrum of 3-methyl-2-butanol. The molecular ion m/z 88 does not appear in...
Related questions
Question
100%
13
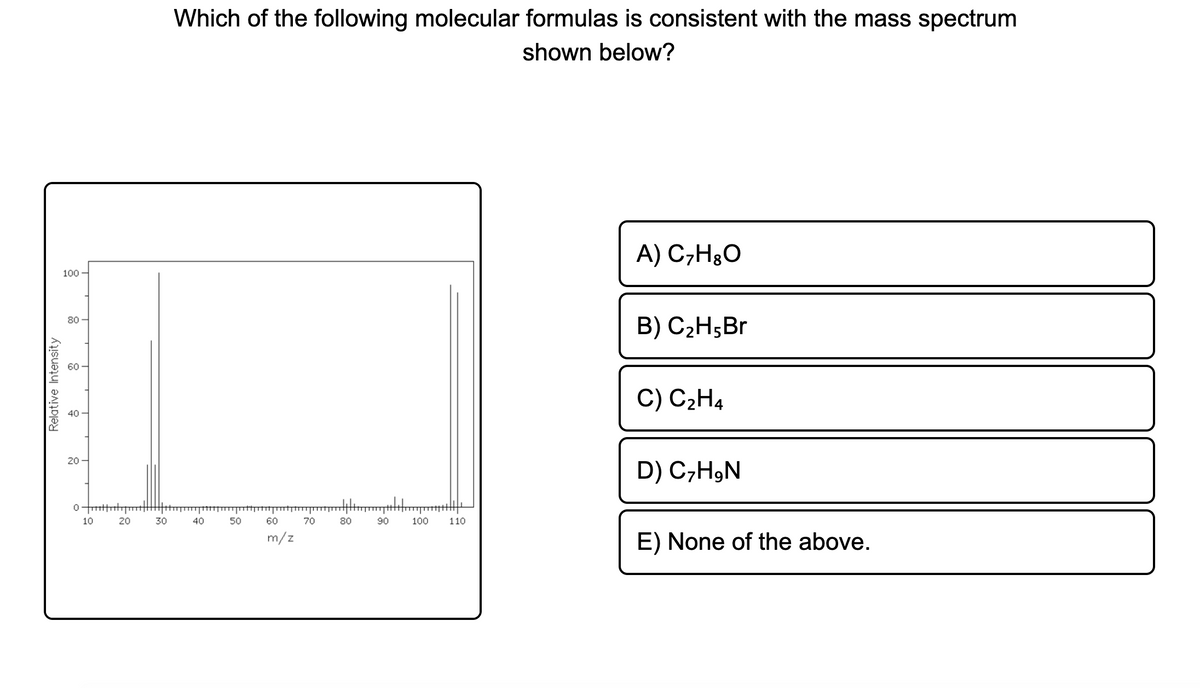
Transcribed Image Text:Which of the following molecular formulas is consistent with the mass spectrum
shown below?
A) C,HgO
100
80
B) C2H5Br
60
C) C2H4
40
20
D) C,H,N
10
20
30
40
50
60
70
80
90
100
110
m/z
E) None of the above.
Relative Intensity
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning