
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14, Problem 14.30P
Following are mass spectra for the constitutional isomers 2-pentanol and 2-methyl- 2-butanol. Assign each isomer its correct spectrum.
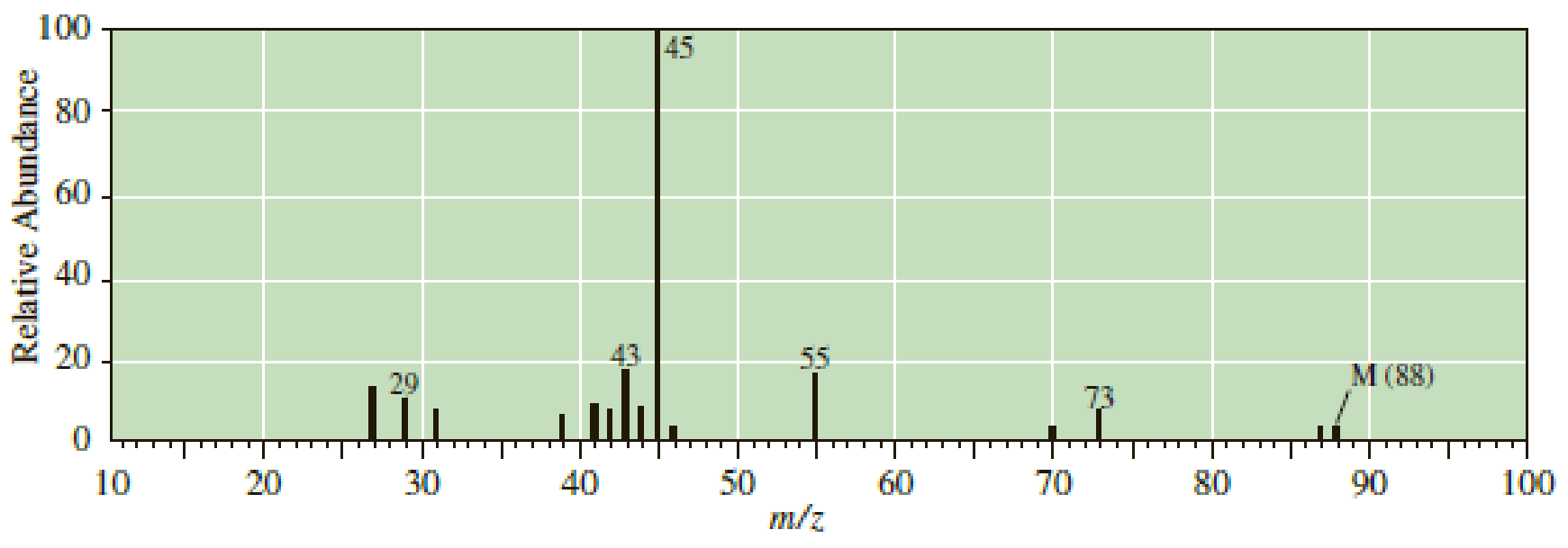
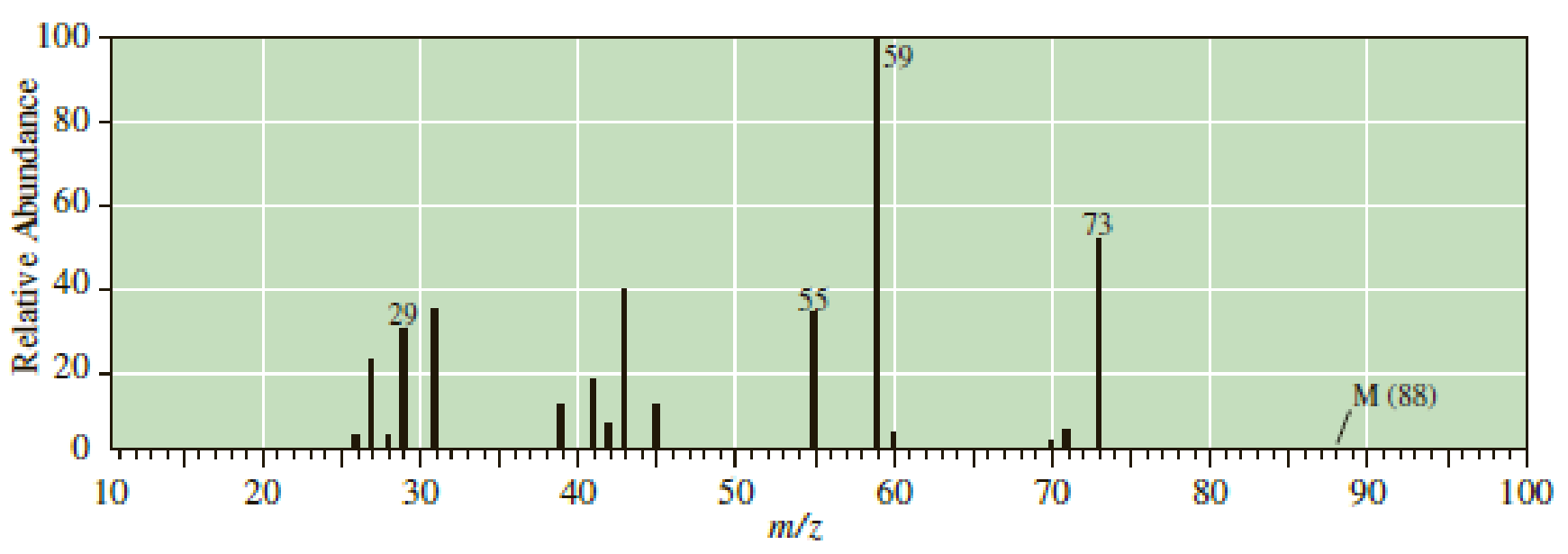
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Determining the Molecular Ions for an Alkyl Chloride
What molecular ions will be present in a mass spectrum of 2-chloropropane, (CH3)2CHCl?
What is the more intense peak of the compound 3-methylpentane in the mass spectrum?
(a) m/z 71 (b) m/z 86 (c) m/z 57 (d) m/z 29
The mass spectrum of an alcohol (C5H12O) exhibits a base peak at m/z =59. Is the alcohol most likely pentan-1-ol, pentan-2-ol, or pentan-3-ol? Explain.
Chapter 14 Solutions
Organic Chemistry
Ch. 14.2 - Calculate the nominal mass of each ion. Unless...Ch. 14.3 - Propose a structural formula for the cation at m/z...Ch. 14.3 - The low-resolution mass spectrum of 2-pentanol...Ch. 14 - Draw acceptable Lewis structures for the molecular...Ch. 14 - The molecular ion for compounds containing only C,...Ch. 14 - For which compounds containing a heteroatom (an...Ch. 14 - The so-called nitrogen rule states that if a...Ch. 14 - Prob. 14.8PCh. 14 - Prob. 14.9PCh. 14 - Prob. 14.10P
Ch. 14 - Determine the probability of the following in a...Ch. 14 - The molecular ions of both C5H10S and C6H14O...Ch. 14 - Prob. 14.13PCh. 14 - Carboxylic acids often give a strong fragment ion...Ch. 14 - For primary amines with no branching on the carbon...Ch. 14 - Prob. 14.16PCh. 14 - A characteristic peak in the mass spectrum of most...Ch. 14 - Predict the relative intensities of the M and M +...Ch. 14 - The mass spectrum of compound A shows the...Ch. 14 - The mass spectrum of compound B, a colorless...Ch. 14 - Write molecular formulas for the five possible...Ch. 14 - Write molecular formulas for the five possible...Ch. 14 - The molecular ion in the mass spectrum of...Ch. 14 - Prob. 14.24PCh. 14 - Following is the mass spectrum of 1-bromobutane....Ch. 14 - Following is the mass spectrum of...Ch. 14 - Following is the mass spectrum of an unknown...Ch. 14 - Following is the mass spectrum of...Ch. 14 - Prob. 14.29PCh. 14 - Following are mass spectra for the constitutional...Ch. 14 - 2-Methylpentanal and 4-methyl-2-pentanone are...Ch. 14 - Prob. 14.32PCh. 14 - Account for the presence of the following peaks in...Ch. 14 - All methyl esters of long-chain aliphatic acids...Ch. 14 - Propylbenzene, C6H5CH2CH2CH3, and isopropyl...Ch. 14 - Account for the formation of the base peaks in...Ch. 14 - Prob. 14.37PCh. 14 - Prob. 14.38P
Additional Science Textbook Solutions
Find more solutions based on key concepts
For each of the following 2-dimensional shapes, determine the highest order rotation axis of symmetry.
Inorganic Chemistry
Q2. Which statement best defines chemistry?
a. The science that studies solvents, drugs, and insecticides
b. Th...
Introductory Chemistry (6th Edition)
Characterize each of the following structures as aromatic, nonaromatic, or antiaromatic:
Answer: _____
Organic Chemistry As a Second Language: Second Semester Topics
Classify each example of molecular art as a pure element, a pure compound, or a mixture.
General, Organic, and Biological Chemistry - 4th edition
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Following are infrared spectra of 2-methyl-1-butanol and tert-butyl methyl ether. Assign each compound its correct spectrum.arrow_forwardFollowing are infrared spectra of nonane and 1-hexanol. Assign each compound its correct spectrum.arrow_forwardFollowing is the mass spectrum of an unknown compound. The two highest peaks are at m/z 120 and 122. Suggest a structure for this compound. (Data from http://webbook.nist.gov/chemistry/.)arrow_forward
- Following is the mass spectrum of 3-methyl-2-butanol. The molecular ion m/z 88 does not appear in this spectrum. Propose structural formulas for the cations of m/z 45, 43, and 41.arrow_forwardPropylbenzene, C6H5CH2CH2CH3, and isopropyl benzene, C6H5CH(CH3)2, are constitutional isomers with the molecular formula C9H12. One of these compounds shows prominent peaks in its mass spectrum at m/z 120 and 105. The other shows prominent peaks at m/z 120 and 91. Which compound has which spectrum?arrow_forwardBelow is the mass spectrum of p-chlorotoluene Identify which peak belongs to the M+ peak/s.arrow_forward
- give the mass spectroscopy interpretation of the peaks Propan-1-amine / Propylaminearrow_forwardWhich of the following molecules matches the given data? (Note: All the molecules have a M+ = m/z 102) C5H10O2 base peak = m/z 43arrow_forwardHow many peaks (13C) would be evident in the decoupled spectrum of a. methylcyclohexane b. cyclohexene c. 1-methylcyclohexene *show structure in the solutionarrow_forward
- Analyze the CNMR for the molecular formula C8H8O3 (vanillin). Propose a table with chemical shift for each peak and assignments of Carbons (assign on structure as well)arrow_forwardIn details explain peaks would you expect to find for t-butyl amine vs. diethyl amine vs. n-butyl amine in the Infrared spectrum?arrow_forwardThe mass spectra of 1-methoxybutane, 2-methoxybutane, and 2-methoxy-2-methylpropane are shown below. Match each compound with its spectrum.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning
IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY