Which of these substances will dissolve in hexane, a non-polar substance? HINT: You will need to determine lines of symmetry and polarity for each molecule. O a Ci-c- Cl: :CI: s=c=s° S=C=S H H H-C-C-H H H
Which of these substances will dissolve in hexane, a non-polar substance? HINT: You will need to determine lines of symmetry and polarity for each molecule. O a Ci-c- Cl: :CI: s=c=s° S=C=S H H H-C-C-H H H
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter23: Carbon: Not Just Another Element
Section: Chapter Questions
Problem 66PS
Related questions
Question
These pictures are connected and I need help finding which ones are polar
(Not honor class)
(Not graded questions)
(An original question)
Transcribed Image Text:ll T-Mobile Wi-Fi ?
9:08 PM
43%
ent/4670075055/assessment
Home-WA ISD 129.
Home | Schoology
- Zoom!
School Emai
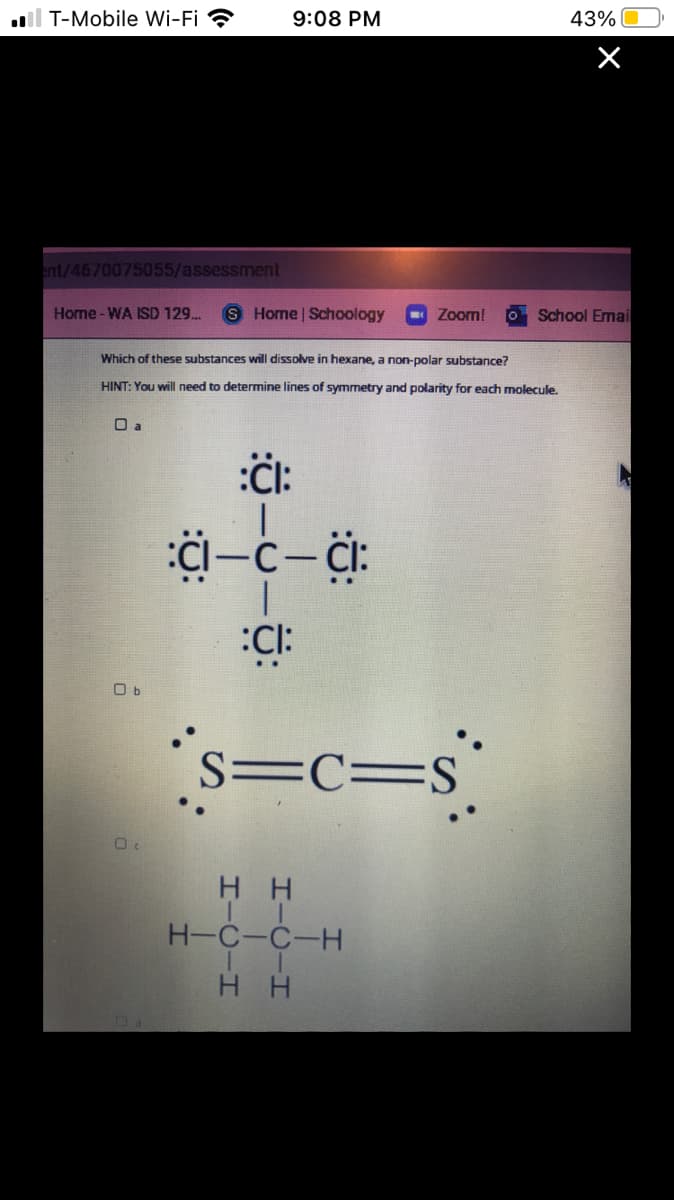
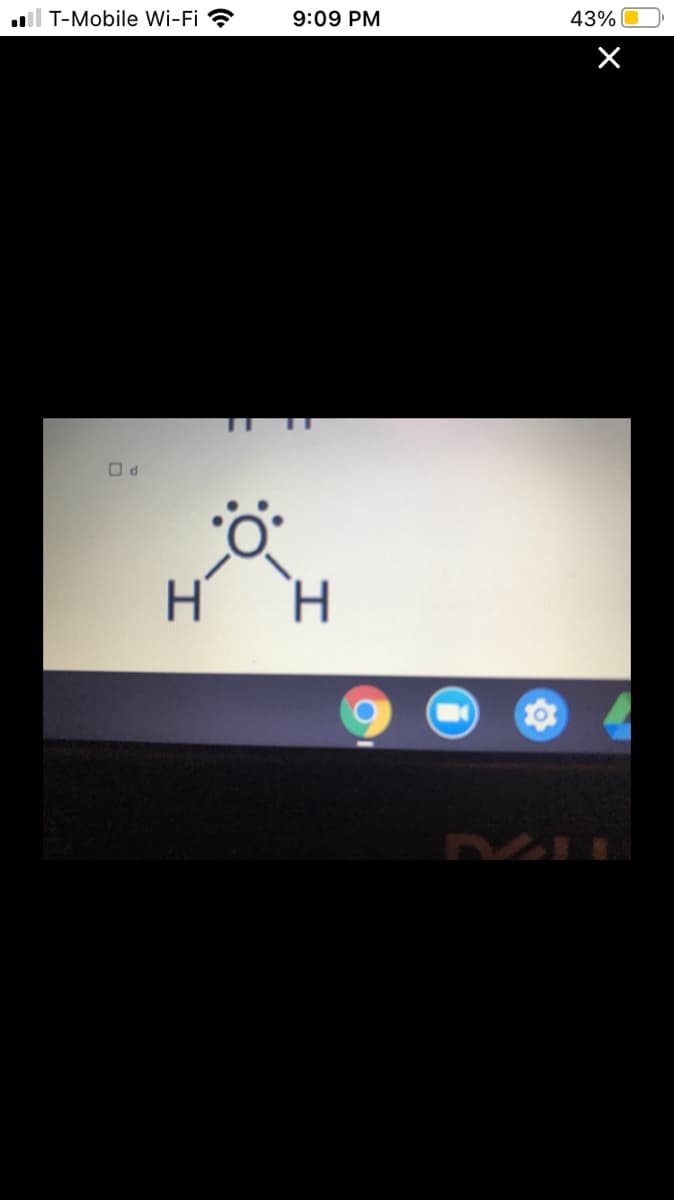
Which of these substances will dissolve in hexane, a non-polar substance?
HINT: You will need to determine lines of symmetry and polarity for each molecule.
O a
:CI:
O b
s=c=s°
S=C=S
нн
H-C-C-H
H H
Transcribed Image Text:ll T-Mobile Wi-Fi ?
9:09 PM
43%
H.
Expert Solution
Step 1

Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning