Which statement about an auxiliary complexing agent is TRUE? O It forms a complex with the metal ion. O It forms a complex with the indicator. O It protects some components in the mixture from reacting with EDTA. OIt increases the conditional formation constant. O It displaces the metal ion from the metal-EDTA complex. A 25.00-mL solution of NaOH was used to titrate 0.786 4 g potassium hydrogen phthalate (KHP, FM 204.2) according to the following reaction. What is the concentration of the NaOH solution? .COOK .COOK NaOH H2O COOH COONA O 0.154 2 M O6.423 M 0.003 855 M O1.542 x 10-2 M O1.542 x 10-4 M
Which statement about an auxiliary complexing agent is TRUE? O It forms a complex with the metal ion. O It forms a complex with the indicator. O It protects some components in the mixture from reacting with EDTA. OIt increases the conditional formation constant. O It displaces the metal ion from the metal-EDTA complex. A 25.00-mL solution of NaOH was used to titrate 0.786 4 g potassium hydrogen phthalate (KHP, FM 204.2) according to the following reaction. What is the concentration of the NaOH solution? .COOK .COOK NaOH H2O COOH COONA O 0.154 2 M O6.423 M 0.003 855 M O1.542 x 10-2 M O1.542 x 10-4 M
Chapter9: Complexometric And Precipitation Titrations
Section: Chapter Questions
Problem 8P
Related questions
Question
3
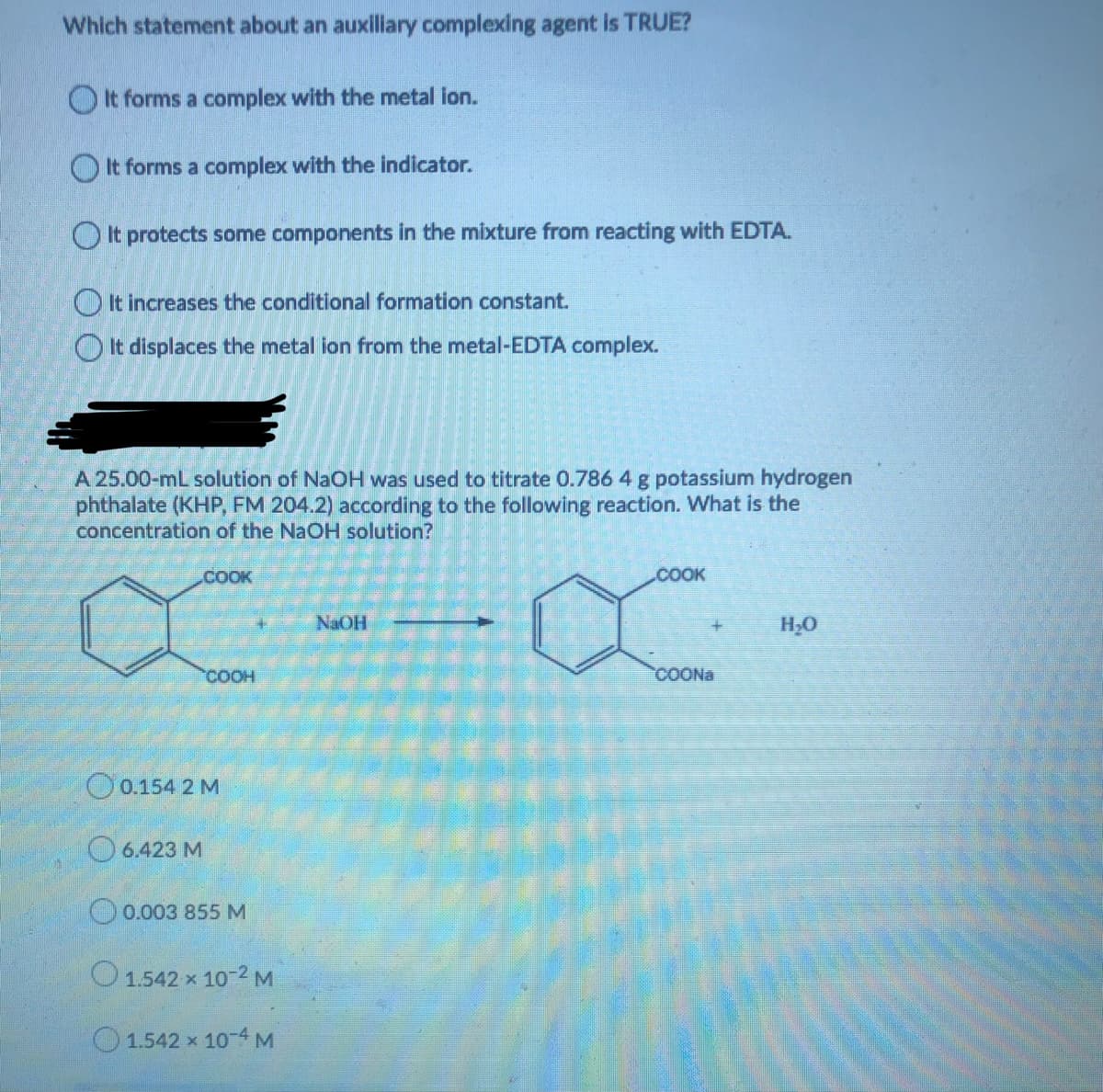
Transcribed Image Text:Which statement about an auxiliary complexing agent is TRUE?
O It forms a complex with the metal ion.
O It forms a complex with the indicator.
OIt protects some components in the mixture from reacting with EDTA.
O It increases the conditional formation constant.
It displaces the metal ion from the metal-EDTA complex.
A 25.00-mL solution of NaOH was used to titrate 0.786 4 g potassium hydrogen
phthalate (KHP, FM 204.2) according to the following reaction. What is the
concentration of the NaOH solution?
.COOK
COOK
NAOH
H;0
COOH
COONA
O0.154 2 M
O 6.423 M
O0.003 855 M
O1.542 x 10-2 M
O1.542 x 10-4 M
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole



Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning