You are in a mountain range with atmospheric air pressure of 520 mmHg, and you wish to boil some eggs. What is the approximate boiling point of the water at this air pressure?
You are in a mountain range with atmospheric air pressure of 520 mmHg, and you wish to boil some eggs. What is the approximate boiling point of the water at this air pressure?
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter15: Gases,liquids, And Solids
Section: Chapter Questions
Problem 107E
Related questions
Question
Transcribed Image Text:I Review | Constants | Periodic Table
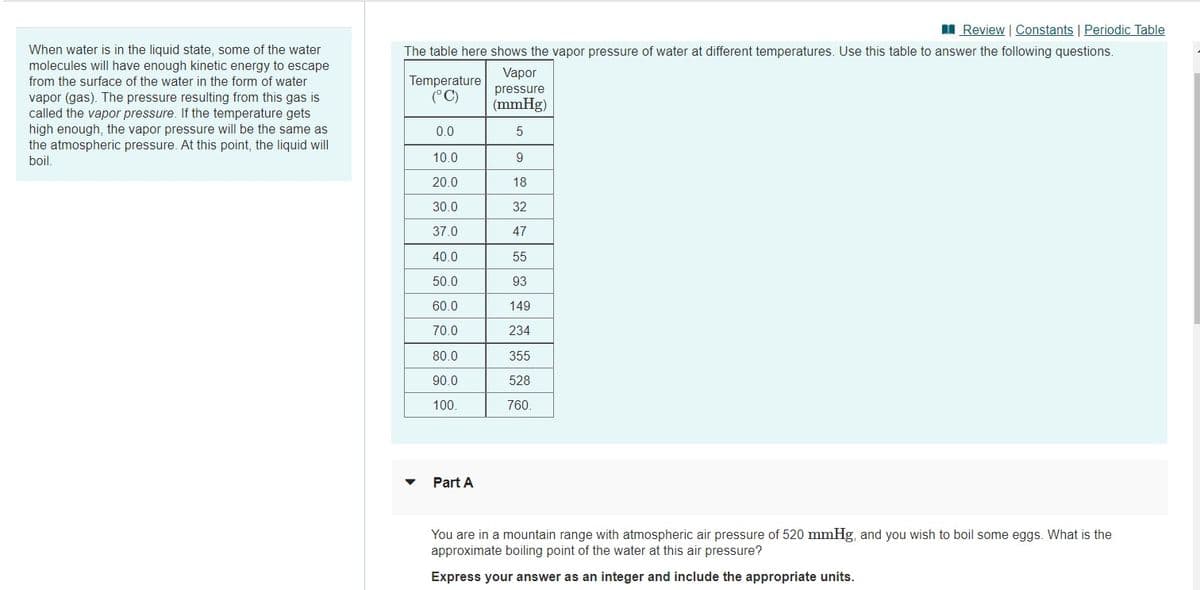
When water is in the liquid state, some of the water
molecules will have enough kinetic energy to escape
The table here shows the vapor pressure of water at different temperatures. Use this table to answer the following questions.
Vapor
Temperature
(°C)
from the surface of the water in the form of water
pressure
vapor (gas). The pressure resulting from this gas is
called the vapor pressure. If the temperature gets
high enough, the vapor pressure will be the same as
the atmospheric pressure. At this point, the liquid will
boil.
(mmHg)
0.0
10.0
20.0
18
30.0
32
37.0
47
40.0
55
50.0
93
60.0
149
70.0
234
80.0
355
90.0
528
100.
760.
Part A
You are in a mountain range with atmospheric air pressure of 520 mmHg, and you wish to boil some eggs. What is the
approximate boiling point of the water at this air pressure?
Express your answer as an integer and include the appropriate units.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co
