
Concept explainers
(a)
Interpretation:TheLewis structure of
Concept Introduction:Lewis dot structure is also known as Lewis dot formula or electron dot structure. The bond formation between the atoms takes place due to the sharing of valence electrons of bonded atoms while the remaining electrons present in outer shell represented as lone pair of electrons.
To draw the Lewis structure, calculate the total number of valence electrons in each atom and draw the structure in such a way that each atom gets its octet configuration.
(a)
Answer to Problem 25P
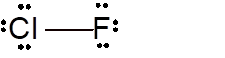
Explanation of Solution
To draw the Lewis structure, calculate total number of valence electrons. Draw these valence electrons and bonded electrons with atoms in such a way that all bonded atoms get octet configuration.
In
Total number of valence electrons =
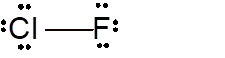
Both Cl and F have octet configuration so there will be no charge on any atom.
(b)
Interpretation: The Lewis structure of
Concept Introduction: Lewis dot structure is also known as Lewis dot formula or electron dot structure. The bond formation between the atoms takes place due to the sharing of valence electrons of bonded atoms while the remaining electrons present in outer shell represented as lone pair of electrons.
To draw the Lewis structure, calculate the total number of valence electrons in each atom and draw the structure in such a way that each atom gets its octet configuration.
(b)
Answer to Problem 25P
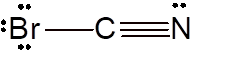
Explanation of Solution
To draw the Lewis structure, calculate total number of valence electrons. Draw these valence electrons and bonded electrons with atoms in such a way that all bonded atoms get octet configuration.
In
Total number of valence electrons =
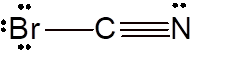
All atoms octet configuration so there will be no charge on any atom.
(c)
Interpretation: The Lewis structure of
Concept Introduction: Lewis dot structure is also known as Lewis dot formula or electron dot structure. The bond formation between the atoms takes place due to the sharing of valence electrons of bonded atoms while the remaining electrons present in outer shell represented as lone pair of electrons.
To draw the Lewis structure, calculate the total number of valence electrons in each atom and draw the structure in such a way that each atom gets its octet configuration.
(c)
Answer to Problem 25P
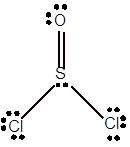
Explanation of Solution
To draw the Lewis structure, calculate total number of valence electrons. Draw these valence electrons and bonded electrons with atoms in such a way that all bonded atoms get octet configuration.
In
Total number of valence electrons =
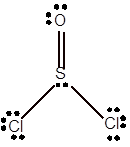
Here S has expanded octet as it has 10 electrons whereas O and Cl has octet configuration.
(d)
Interpretation: The Lewis structure of
Concept Introduction: Lewis dot structure is also known as Lewis dot formula or electron dot structure. The bond formation between the atoms takes place due to the sharing of valence electrons of bonded atoms while the remaining electrons present in outer shell represented as lone pair of electrons.
To draw the Lewis structure, calculate the total number of valence electrons in each atom and draw the structure in such a way that each atom gets its octet configuration.
(d)
Answer to Problem 25P
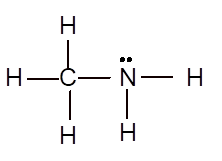
Explanation of Solution
To draw the Lewis structure, calculate total number of valence electrons. Draw these valence electrons and bonded electrons with atoms in such a way that all bonded atoms get octet configuration.
In
Total number of valence electrons =
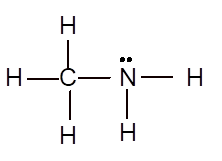
All atoms have octet configuration.
(e)
Interpretation: The Lewis structure of
Concept Introduction: Lewis dot structure is also known as Lewis dot formula or electron dot structure. The bond formation between the atoms takes place due to the sharing of valence electrons of bonded atoms while the remaining electrons present in outer shell represented as lone pair of electrons.
To draw the Lewis structure, calculate the total number of valence electrons in each atom and draw the structure in such a way that each atom gets its octet configuration.
(e)
Answer to Problem 25P
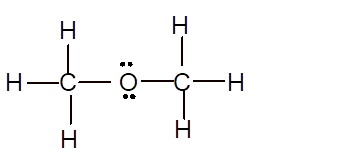
Explanation of Solution
To draw the Lewis structure, calculate total number of valence electrons. Draw these valence electrons and bonded electrons with atoms in such a way that all bonded atoms get octet configuration.
In
Total number of valence electrons =
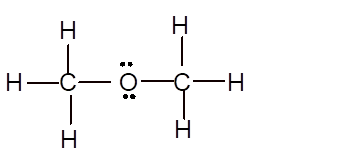
All atoms have octet configuration.
(f)
Interpretation: The Lewis structure of
Concept Introduction: Lewis dot structure is also known as Lewis dot formula or electron dot structure. The bond formation between the atoms takes place due to the sharing of valence electrons of bonded atoms while the remaining electrons present in outer shell represented as lone pair of electrons.
To draw the Lewis structure, calculate the total number of valence electrons in each atom and draw the structure in such a way that each atom gets its octet configuration.
(f)
Answer to Problem 25P
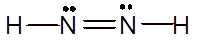
Explanation of Solution
To draw the Lewis structure, calculate total number of valence electrons. Draw these valence electrons and bonded electrons with atoms in such a way that all bonded atoms get octet configuration.
In
Total number of valence electrons =
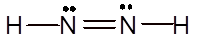
All atoms have octet configuration.
(g)
Interpretation: The Lewis structure of
Concept Introduction: Lewis dot structure is also known as Lewis dot formula or electron dot structure. The bond formation between the atoms takes place due to the sharing of valence electrons of bonded atoms while the remaining electrons present in outer shell represented as lone pair of electrons.
To draw the Lewis structure, calculate the total number of valence electrons in each atom and draw the structure in such a way that each atom gets its octet configuration.
(g)
Answer to Problem 25P
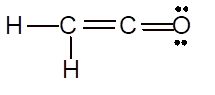
Explanation of Solution
To draw the Lewis structure, calculate total number of valence electrons. Draw these valence electrons and bonded electrons with atoms in such a way that all bonded atoms get octet configuration.
In
Total number of valence electrons =
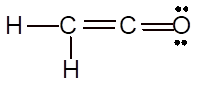
All atoms have octet configuration.
(h)
Interpretation: The Lewis structure of
Concept Introduction: Lewis dot structure is also known as Lewis dot formula or electron dot structure. The bond formation between the atoms takes place due to the sharing of valence electrons of bonded atoms while the remaining electrons present in outer shell represented as lone pair of electrons.
To draw the Lewis structure, calculate the total number of valence electrons in each atom and draw the structure in such a way that each atom gets its octet configuration.
(h)
Answer to Problem 25P
Explanation of Solution
To draw the Lewis structure, calculate total number of valence electrons. Draw these valence electrons and bonded electrons with atoms in such a way that all bonded atoms get octet configuration.
In
Total number of valence electrons =
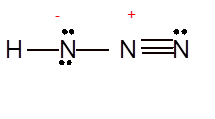
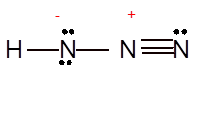
(i)
Interpretation: The Lewis structure of
Concept Introduction: Lewis dot structure is also known as Lewis dot formula or electron dot structure. The bond formation between the atoms takes place due to the sharing of valence electrons of bonded atoms while the remaining electrons present in outer shell represented as lone pair of electrons.
To draw the Lewis structure, calculate the total number of valence electrons in each atom and draw the structure in such a way that each atom gets its octet configuration.
(i)
Answer to Problem 25P
Explanation of Solution
To draw the Lewis structure, calculate total number of valence electrons. Draw these valence electrons and bonded electrons with atoms in such a way that all bonded atoms get octet configuration.
In
Total number of valence electrons =
Want to see more full solutions like this?
Chapter 1 Solutions
ORGANIC CHEM F/UT (LOOSELEAF)
- Describe the molecular structure around the indicated atom or atoms: (a) the sulfur atom in sulfuric acid, H2SO4[(HO)2SO2] (b) the chlorine atom in chloric acid, HClO3[HOClO2] (c) the oxygen atom in hydrogen peroxide, HNO3[HONO2] (d) the nitrogen atom in nitric acid, HNO3[HONO2] (e) the oxygen atom in the OH group in nitric acid, HNO3[HONO2] (f) the central oxygen atom in the ozone molecule, O3 (g) each of the carbon atoms in propyne, CH3CCH (h) the carbon atom in Freon, CCl2F2 (i) each of the carbon atoms in aliene, H2CCH2arrow_forwardCompare your answers from parts a and b of Exercise 69 with H values calculated for each reaction using standard enthalpies of formation in Appendix 4. Do enthalpy changes calculated from bond energies give a reasonable estimate of the actual values?arrow_forwardLewis structures can be used to understand why some molecules react in certain ways. Write the Lewis structures for the reactants and products in the reactions described below. a. Nitrogen dioxide dimerizes to produce dinitrogen tetroxide. b. Boron trihydride accepts a pair of electrons from ammonia, forming BH3NH3. Give a possible explanation for why these two reactions occur.arrow_forward
- Consider the following Lewis structure where E is an unknown element: What are some possible identities for element E? Predict the molecular structure (including bond angles) for this ion. (See Exercises 115 and 116.)arrow_forwardDraw resonance structures for the SO2 molecule, and determine the formal charges on the S and O atoms. Are the SO bonds polar, and is the molecule as a whole polar? If so, what is the direction of the net dipole in SO2? Is your prediction confirmed by the electrostatic potential surface? Explain briefly.arrow_forwardAn important observation supporting the concept of resonance in the localized electron model was that there are only three different structures of dichlorobenzene (C6H4C10). How does this fact support the concept of resonance (see Exercise 89)?arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning





