
CHEMISTRY:CENTRAL SCI.W/MASTERINGCHEM
17th Edition
ISBN: 9781323738429
Author: Brown
Publisher: PEARSON C
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 4E
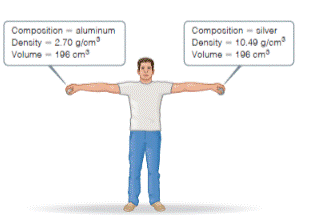
Consider the two spheres shown here, one made of silver and the other of aluminum.
- What is the mass of each sphere in kg?
- The force of gravity acting on an object is F = mg, where m is the mass of an object and g is the acceleration of gravity (9.8 m/s2). How much work do you do on each sphere it you raise it from the floor to a height of 2.2 m?
- Does the act of lifting the sphere off the ground increase the potential energy of the aluminum sphere by a larger, smaller, or same amount as the silver sphere?
- If you release the spheres simultaneously, they will have the same velocity when they hit the ground. Will they have the same kinetic energy? If not, which sphere will have more kinetic energy? [Section 1.4 Q]
Expert Solution & Answer
Learn your wayIncludes step-by-step video

schedule04:21
Students have asked these similar questions
A subcompact car with a mass of 1.60×103 kg and a loaded dump truck with a mass of 1.60×104 kg are traveling at the same speed.
How many times more kinetic energy does the dump truck have than the car?
5. A typical recipe for a hot toddy (a drink often used as a cold remedy) is to mix hot water with whiskey, honey, and lemon juice. A patient heats 16 oz. of 22 o C water on the stove until it has absorbed 33 kcal of energy. He then mixes 2.0 oz. of the hot water with 1.5 oz. of room temperature whiskey (d = 0.94 g/cm3 , cs = 3.40 J/g o C, T = 22 o C). What is the temperature of the hot toddy (prior to adding the remaining ingredients)?
Which of the following is a physical property?
Which of the following items is a pure substance?
What is the value of 98 °F in units of °C?
Convert 68852 millijoules into Calories. (Write your answer in the decimal form?
When table salt is placed in water and stirred it dissolves and a clear liquid is obtained. This process is a:
Some groceries are placed in a deep freezer that is set at 18 oF. What is this temperature in Celsius?
Thermal energy is:
Which of the following statements is true about a compound?
How many joules are there in a 255 Calorie snack bar?
Which of the following is a physical property?
If the monthly electricity consumption of a household is 6,619 kWh, what is this consumption expressed in kJ?
Which of the following items is a chemical property?
If you hold a solid piece of pure gallium metal in your hand, your body heat will melt the gallium into its liquid form. This illustrates which of the following?
On a cold day the atmospheric…
Chapter 1 Solutions
CHEMISTRY:CENTRAL SCI.W/MASTERINGCHEM
Ch. 1.2 - Practice Exercise 1 Which of the following is the...Ch. 1.2 - Aspirin is composed of 60.0% carbon, 4.5%...Ch. 1.5 - Practice Exercise 1 Which of the following weights...Ch. 1.5 - Practice Exercise 2 How many picometers are there...Ch. 1.5 - Practice Exercise 1 Using Wolfram Alpha...Ch. 1.5 - Practice Exercise 2 Ethylene glycol, the major...Ch. 1.5 - Practice Exercise 1 Platinum, Pt. is one of the...Ch. 1.5 - Practice Exercise 2 Calculate the density of a...Ch. 1.5 - Which of the following objects has the greatest...Ch. 1.5 - Prob. 1.5.2PE
Ch. 1.6 - Which of the following numbers in your personal...Ch. 1.6 - Practice Exercise 2 The back inside cover of the...Ch. 1.6 - Practice Exercise 1 An object is determined to...Ch. 1.6 - Practice Exercise 2 How many significant figures...Ch. 1.6 - Ellen recently purchased a new hybrid car and...Ch. 1.6 - Practice Exercise 2 It takes 10.5 s for a sprinter...Ch. 1.6 - Practice Exercise 1 You are asked to determine the...Ch. 1.6 - Practice Exercise 1 At a particular instant in...Ch. 1.7 - Practice Exercise 2 By using a conversion factor...Ch. 1.7 - Practice Exercise 1 Fabiola, who lives in Mexico...Ch. 1.7 - Practice Exercise 2 A car travels 28 mi per gallon...Ch. 1.7 - Practice Exercise 2 A car travels 28 mi per gallon...Ch. 1.7 - Practice Exercise 2 The surface area of Earth is...Ch. 1.7 - Practice Exercise 1 Composite decking is a...Ch. 1.7 - Practice Exercise 2 The density of the organic...Ch. 1.7 - Practice Exercise 2 If the mass of the container...Ch. 1 - Prob. 1ECh. 1 - Prob. 2ECh. 1 - Musical instruments like trumpets and trombones...Ch. 1 - Consider the two spheres shown here, one made of...Ch. 1 - Is the separation method used in brewing a cup of...Ch. 1 - Identify each of the following as measurements of...Ch. 1 - Three spheres of equal size are composed of...Ch. 1 - The three targets from a rifle range shown below...Ch. 1 - What is the length of the pencil in the following...Ch. 1 - How many significant figures should be reported...Ch. 1 - Consider the jar of jelly beans in the photo. To...Ch. 1 - The photo below shows a picture of an agate stone....Ch. 1 - Classify each of the following as a pure substance...Ch. 1 - Classify each of the following as a pure substance...Ch. 1 - 1.15 Give the chemical symbol or name for the...Ch. 1 - 1.16 Give the chemical symbol or name for each of...Ch. 1 - A solid white substance A is heated strongly in...Ch. 1 - 1.18 You are hiking in the mountains and find a...Ch. 1 - 1.19 In the process of attempting to characterize...Ch. 1 - 1.20
Read the following description of the element...Ch. 1 - Prob. 21ECh. 1 - A match is lit and held under a cold piece of...Ch. 1 - Which separation method is better suited for...Ch. 1 - Two beakers contain clear, colorless liquids. When...Ch. 1 - Prob. 25ECh. 1 - Prob. 26ECh. 1 - Prob. 27ECh. 1 - Prob. 28ECh. 1 - Prob. 29ECh. 1 - Prob. 30ECh. 1 - 121 What exponential notation do the following...Ch. 1 -
1.32 Use appropriate metric prefixes to write the...Ch. 1 - Make the following conversions. 72 °F to °C, 216.7...Ch. 1 - a. The temperature on a warm summer day is 87 °F....Ch. 1 - Prob. 35ECh. 1 - A cube of osmium metal 1.500 cm on a side has a...Ch. 1 - To identify a liquid substance, a student...Ch. 1 - a. After the label fell off a bottle containing a...Ch. 1 - Prob. 39ECh. 1 -
1.40 Silicon for computer chips is grown in large...Ch. 1 - Prob. 41ECh. 1 - 1.42 A watt is a measure of power (the rate of...Ch. 1 - Indicate which of the following are exact numbers;...Ch. 1 - Indicate which of the following are exact numbers:...Ch. 1 - 1.45 What is the number of significant figures in...Ch. 1 - Indicate the number of significant figures in each...Ch. 1 - 1.47 Round each of the following numbers to four...Ch. 1 - 1.48
The diameter of Earth at the equator is 7926...Ch. 1 - Carry out the following operations and express the...Ch. 1 - Carry out the following operations and express the...Ch. 1 - You weigh an object on a balance and read the mass...Ch. 1 - You have a graduated cylinder that contains a...Ch. 1 - 153 Using your knowledge of metric units, English...Ch. 1 - 1.54 Using your knowledge of metric units, English...Ch. 1 - A bumblebee flies with a ground speed of 15.2 m/s....Ch. 1 - 1 56
a The speed of light in a vacuum is 2.998 x...Ch. 1 - Perform the following conversions: 5.00 days to s,...Ch. 1 - Carry out the following conversions: 0.105 in. to...Ch. 1 - How many liters of wine can be held in a wine...Ch. 1 - If an electric car is capable of going 225 km on a...Ch. 1 - The density of air at ordinary atmospheric...Ch. 1 - 1.62 The concentration of carbon monoxide in an...Ch. 1 - Prob. 63ECh. 1 - 1.64 A copper refinery produces a copper ingot...Ch. 1 - 165 Classify ea. al the folbwing as a pure...Ch. 1 - 1.66
Which is more likely to eventually be shown...Ch. 1 -
1.67 A sample of ascorbic acid (vitamin C) is...Ch. 1 - Prob. 68AECh. 1 - SO Two students deterrmne the percen.ge of lead in...Ch. 1 - 1.70
Is Om use of significant figures in ea. of...Ch. 1 - What type of quantity (for example, length,...Ch. 1 - 1.72 Give the derived SI units for each of the...Ch. 1 - 1.73 The distance from Earth to the Moon is...Ch. 1 - 1.74 Which of the following would you characterize...Ch. 1 -
1.75 The U.S. quarter has a mass of 5.67 g and is...Ch. 1 -
1.76 In the United States, water used for...Ch. 1 -
1.77 By using estimation techniques, determine...Ch. 1 - Suppose you decide to define your own temperature...Ch. 1 -
1.79 The liquid substances mercury (density =...Ch. 1 -
1.80 Two spheres of equal volume are placed on...Ch. 1 - Water has a density of 0.997 g/cm3 at 25C ; ice...Ch. 1 - A 32.65-g sample of a solid is placed in a flask....Ch. 1 - A thief plans to steal a gold sphere with a radius...Ch. 1 - Automobile batteries contain sulfuric acid, which...Ch. 1 - A 40-lb container of peat moss measures 14 x 20 x...Ch. 1 - A package of aluminum foil contains 50 ft2of foil,...Ch. 1 - Prob. 87AECh. 1 -
1.88 In 2005, J. Robin Warren and Barry J....Ch. 1 -
1 89 A 25 0-cm.long cylindrical glass tube,...Ch. 1 -
1.90 Gold is alloyed (mixed) with other metals to...Ch. 1 -
1.91 Paper chromatography is a simple but...Ch. 1 -
1.92 Judge the following statements as true or...Ch. 1 -
1.93 You are assigned the task of separating a...Ch. 1 - Prob. 94AE
Additional Science Textbook Solutions
Find more solutions based on key concepts
11.57 Draw the cis and trans isomers for each of the following: (11.6)
a. 2-pentene
b. 3-hexene
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
45. The tabulated data were collected for this reaction:
[NO2](M) [F2](M) Initial Rate (M/s)
0.100 0.100...
Chemistry: Structure and Properties
Calculate the lattice energy of CaCl2 using a Born-Haber cycle and data from Appendices F and L and Table 7.5. ...
Chemistry & Chemical Reactivity
Two samples of a compound containing elements A and B are decomposed. The first sample produced15 g of A and 35...
Chemistry: A Molecular Approach
a. Prepare a molecular orbital energy-level diagram for NO showing clearly how the atomic orbitals interact to ...
Inorganic Chemistry
9.1 Calculate the total mass of the reactants and the products for each of the following equations:
Basic Chemistry (5th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1-86 The specific heats of some elements at 25oC are as follows: aluminum = 0.215 cal/g · oC; carbon (graphite) = 0.170 caI/g oC; iron = 0.107 cal/g mercury = 0.033 1 caI/g oC. (a) Which element would require the smallest amount of heat to raise the temperature of 100 g of the element by 10oC? (b) If the same amount of heat needed to raise the temperature of 1 g of aluminum by 25oC were applied to 1 g of mercury, by how many degrees would its temperature be raised? (c) If a certain amount of heat is used to raise the temperature of 1.6 g of iron by 10oC, the temperature of 1 g of which element would also be raised by 10oC, using the same amount of heat?arrow_forwardThe following data refer to the element phosphorus. Classify each as a physical or a chemical property. (a) It exists in several forms, for example, white, black, and red phosphorus. (b) It is a solid at 25C and 1 atm. (c) It is insoluble in water. (d) It burns in chlorine to form phosphorus trichloride.arrow_forwardA 124-g sample of a pure liquid, liquid A, with a density of 3.00 g/mL is mixed with a 40.8-mL sample of a pure liquid, liquid B, with a density of 2.00 g/mL. What is the total volume of the mixture? (Assume there is no reaction upon the mixing of A and B, and volumes are additive.)arrow_forward
- The average adult human burns 2.00 × 10³ kcal per day in energy. What is this rate in kJ per hour? Please use a conversion table to show the work.arrow_forwardThe SI unit for energy is the joule (J), which is equal to the kineticenergy possessed by a 2.00-kg mass moving at 1.00 m/s. Convertthis velocity to mph (1 mi = 1.609 km).(a) 4.47 × 10–7 mph (b) 5.79 × 106 mph (c) 5.79 mph (d) 0.0373 mph (e) 2.24 mpharrow_forwardCarry out each of the following calculations, paying special attention to significant figures, rounding, and units (J = joule, the SI unit of energy; mol = mole, the SI unit for amount of substance) (6.022 X 1 023 molecules/mol)( 1 .l 9 X 1 02 g) / 46.07 glmolarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning
Measurement and Significant Figures; Author: Professor Dave Explains;https://www.youtube.com/watch?v=Gn97hpEkTiM;License: Standard YouTube License, CC-BY
Trigonometry: Radians & Degrees (Section 3.2); Author: Math TV with Professor V;https://www.youtube.com/watch?v=U5a9e1J_V1Y;License: Standard YouTube License, CC-BY