
Organic Chemistry, Books a la Carte Edition and Study Guide and Student's Solutions Manual for Organic Chemistry (7th Edition)
7th Edition
ISBN: 9780133903652
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10, Problem 48P
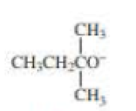
When 2-bromo-2,3-dimethylbutane reacts with a strong base, two
- a. Which of the bases (A, B, C, or D) would form the highest percentage of the highest percentage of the 1-alkene?
- b. Which would give the highest percentage of the 2-alkene?
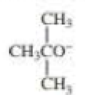
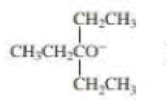
- A. CH3CH2O–
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
1. Which reaction conditions would be best for turning ethene into ethanol?
a.H2O, HBr
b. CH3OH, H2SO4
c.CH3OH, HBR
d. H2O, H2SO4
2. Which alkene would be non-regioselective in a reaction with HBr?
a. 2-ehyl-2-methyl-2-butene
b.2-ethyl-3-methyl-2-butene
c. 2,3-dimethyl-2-butene
d.1,2-dimethyl-2-butene
1. What type of reaction is occuring in step 3? (halogenation, hydrohalogenation, reduction, keto–enol tautomerism, dehydrohalogenation, acid-catalyzed hydration, base-catalyzed hydration)
2. Which reagent is necessary for step 3? (Br2, HBr, H2/Pt, NaNH2, H20/H2SO4/HgSO4)
In each case tell which SN2 reaction will proceed faster.1. The displacement by I- on (a) CH3Cl or (b) CH3OTos.
Select A or B2. The displacement by OH- on (a) CH3Br or (b) CH3I.
Select A or B
Chapter 10 Solutions
Organic Chemistry, Books a la Carte Edition and Study Guide and Student's Solutions Manual for Organic Chemistry (7th Edition)
Ch. 10.2 - Prob. 1PCh. 10.2 - Prob. 2PCh. 10.2 - Prob. 3PCh. 10.2 - Prob. 4PCh. 10.3 - Four alkenes are formed from the E1 reaction of...Ch. 10.3 - If 2-fluoropentane could undergo an E1 reaction,...Ch. 10.3 - Prob. 7PCh. 10.3 - Propose a mechanism for the following reaction:Ch. 10.4 - Prob. 9PCh. 10.4 - What products will be obtained from the El...
Ch. 10.4 - Prob. 11PCh. 10.5 - Prob. 12PCh. 10.6 - Prob. 14PCh. 10.7 - Why do cis-1-bromo-2-ethylcyclohexane and...Ch. 10.7 - Which isomer reacts more rapidly in an E2...Ch. 10.7 - Prob. 18PCh. 10.8 - Prob. 19PCh. 10.8 - Prob. 20PCh. 10.9 - Prob. 21PCh. 10.9 - Explain why only a substitution product and no...Ch. 10.9 - Prob. 23PCh. 10.9 - Prob. 24PCh. 10.9 - Prob. 25PCh. 10.9 - a. Explain why 1-bromo-2,2-dimethylpropane has...Ch. 10.10 - A small amount of another organic product is...Ch. 10.10 - What is the best way to prepare the following...Ch. 10.10 - Prob. 29PCh. 10.10 - Prob. 30PCh. 10.10 - Why is a cumulated diene not formed in the...Ch. 10.10 - What product is obtained when the following...Ch. 10.11 - Prob. 33PCh. 10.11 - Prob. 34PCh. 10 - Draw the major product obtained when each of the...Ch. 10 - Prob. 36PCh. 10 - a. Indicate how each of the following factors...Ch. 10 - Prob. 38PCh. 10 - A chemist wanted to synthesize the...Ch. 10 - Prob. 40PCh. 10 - Prob. 41PCh. 10 - Prob. 42PCh. 10 - Starting with an alkyl halide, how could the...Ch. 10 - Indicate which species in each pair gives a higher...Ch. 10 - Prob. 45PCh. 10 - For each of the following alkyl halides, indicate...Ch. 10 - Prob. 47PCh. 10 - When 2-bromo-2,3-dimethylbutane reacts with a...Ch. 10 - Prob. 49PCh. 10 - When the following compound undergoes solvolysis...Ch. 10 - cis-1-Bromo-4-tert-butylcyclohexane and...Ch. 10 - Draw the substitution and elimination products.Ch. 10 - Prob. 53PCh. 10 - Prob. 54PCh. 10 - Which of the following hexachlorocyclohexanes is...Ch. 10 - Explain why the rate of the reaction of...Ch. 10 - Prob. 57PCh. 10 - Two elimination products are obtained from the...Ch. 10 - Draw the structures or the product of the obtained...Ch. 10 - How could you prepare the following compounds from...Ch. 10 - cis-4-Bromocyclohexanol and...Ch. 10 - Prob. 62PCh. 10 - Prob. 63P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What reagents would you use to prepare the next alkenearrow_forwardReaction of (CH3)3CH with Cl2 forms two products: (CH3)2CHCH2Cl (63%) and (CH3)3CCl (37%). Why is the major product formed by cleavage of the stronger 1° C–H bond?arrow_forward1 Consider the reaction of (R)-2-chloro-3-methylbutane with sodium iodide to form aproduct.(a) Draw the reaction scheme with the correct stereochemistry (reactant + NaI → product+ NaCl). Circle the nucleophile and draw a rectangle around the electrophile.(b) What is the symbol used for mechanism shown in 1(a)(c) If the sodium iodide was replaced with sodium hydroxide, the product is anALKENE. Draw a reaction MECHANISM to show how this happens.(d) Draw the reaction energy diagram for the reaction in 1(c) and label the activationenergy. (e) Using any alcohol with five carbons, and any carboxylic acid with six carbons, draw areaction to show how we would make an ester.(f) Describe the practical on esters. Please answer (d) to (f)arrow_forward
- #20 B Draw structural formulas for all possible carbocations formed by the reaction of each alkene with HCl.arrow_forwardDraw the major products obtained from the reaction of one equivalent of HCl with the following compounds. For each reaction, indicate the kinetic andthermodynamic products. a. 2,3-dimethyl-1,3-pentadiene b. 2,4-dimethyl-1,3-pentadienearrow_forwarda. Identify two alkenes that react with HBr to form 1-bromo-1-methylcyclohexane without undergoing a carbocation rearrangement. b. Would both alkenes form the same alkyl halide if DBr were used instead of HBr? (D is an isotope of H, so D+ reacts like H+.)arrow_forward
- Consider the reaction of (R)-2-chloro-3-methylbutane with sodium iodide to form aproduct.1(a) Draw the reaction scheme with the correct stereochemistry (reactant + NaI → product+ NaCl). Circle the nucleophile and draw a rectangle around the electrophile. 1(b) What is the symbol used for mechanism shown in 1(a)?1(c) If the sodium iodide was replaced with sodium hydroxide, the product is anALKENE. Draw a reaction MECHANISM to show how this happens.1(d) Draw the reaction energy diagram for the reaction in 1(c) and label the activationenergy.1(e) Using any alcohol with five carbons, and any carboxylic acid with six carbons, draw areaction to show how we would make an ester. 1(f) Describe the practical on esters.arrow_forwardhow did you get the answer for part a: (s)-butan-2-ol , and how did you get the answer for part barrow_forwardTRUE OR FALSE and explain why 1. HBr is a by-product of reaction of alkenes with bromine (electrophilic addition)2. Alkynes have 2 pi bonds: C-H and C-C3. Ammoniacal AgNO3 Test is a general confirmatory test for alkynesarrow_forward
- What is the limiting reagent, and their ratios to each other? ( example: 2:3:1) Bromobenzene: 4.5mL Magnesium:1.0g Methyl benzoate:2.5mL Product: 1.82garrow_forward3 Organic Chemistry, helppp with ALL PARTS(A&B) Consider the monoterpene linalool, a natural product used in the fragrance industry. When linalool (right panel) is treated with a strong protic acid in the presence of heat, 12 distinct products form. a. Given the initial structure of linalool, draw the structure of these 12 products b. Show the structure of the five intermediates that facilitate the formation of these 12 products and use arrow pushing to show how the intermediates formarrow_forwardDraw the product or products that will be obtained from the reaction of cis-2-butene and trans-2-butene with each of the following reagents. If a product can exist as stereoisomers, show which stereoisomers are formed. 1. HCl. 4.Br2 in CH2Cl2 7. H2O + H2SO4 2. BH3/THF, followed by HO-, H2O2, H2O 5. Br2 + H2O 8. CH3OH + H2SO4 3. a peroxyacid 6. H2 + Pd/Carrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
How to Design a Total Synthesis; Author: Chemistry Unleashed;https://www.youtube.com/watch?v=9jRfAJJO7mM;License: Standard YouTube License, CC-BY