
(a)
Interpretation:
The 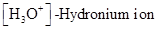 and
and 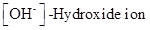 values of given solution should be determined and the most acidic and basic solution should be identified.
values of given solution should be determined and the most acidic and basic solution should be identified.
Concept Introduction:
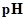 : The concentration of hydrogen ion is measured using
: The concentration of hydrogen ion is measured using 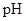 scale. The
scale. The 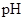 of a solution is a figure that expresses the acidity or the alkalinity of a given solution.
of a solution is a figure that expresses the acidity or the alkalinity of a given solution.
It is defined as the negative base-10 logarithm of the hydrogen or hydronium ion concentration.
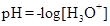
If the value of 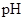 is less than
is less than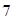 , then the solution is acidic whereas if the value of
, then the solution is acidic whereas if the value of 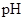 is greater than
is greater than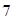 , then the solution is basic.
, then the solution is basic.
Ionic-product constant for water: It is the hydronium ion concentration times the 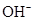 concentration present in the solution.
concentration present in the solution.
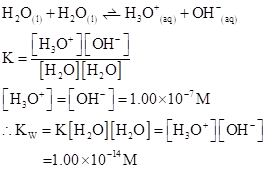
The 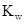 will apply to all aqueous solution.
will apply to all aqueous solution.
For acidic solution 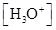 is large that is
is large that is 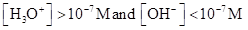
For basic solution 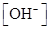 is large that is
is large that is 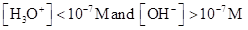
(b)
Interpretation:
The 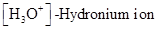 and
and 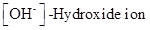 values of given solution should be determined and the most acidic and basic solution should be identified.
values of given solution should be determined and the most acidic and basic solution should be identified.
Concept Introduction:
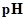 : The concentration of hydrogen ion is measured using
: The concentration of hydrogen ion is measured using 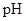 scale. The
scale. The 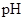 of a solution is a figure that expresses the acidity or the alkalinity of a given solution.
of a solution is a figure that expresses the acidity or the alkalinity of a given solution.
It is defined as the negative base-10 logarithm of the hydrogen or hydronium ion concentration.
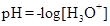
If the value of 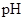 is less than
is less than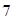 , then the solution is acidic whereas if the value of
, then the solution is acidic whereas if the value of 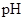 is greater than
is greater than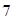 , then the solution is basic.
, then the solution is basic.
Ionic-product constant for water: It is the hydronium ion concentration times the 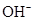 concentration present in the solution.
concentration present in the solution.
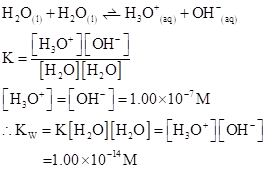
The 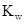 will apply to all aqueous solution.
will apply to all aqueous solution.
For acidic solution 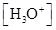 is large that is
is large that is 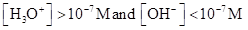
For basic solution 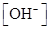 is large that is
is large that is 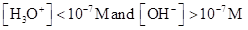
(c)
Interpretation:
The 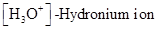 and
and 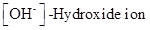 values of given solution should be determined and the most acidic and basic solution should be identified.
values of given solution should be determined and the most acidic and basic solution should be identified.
Concept Introduction:
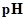 : The concentration of hydrogen ion is measured using
: The concentration of hydrogen ion is measured using 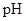 scale. The
scale. The 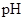 of a solution is a figure that expresses the acidity or the alkalinity of a given solution.
of a solution is a figure that expresses the acidity or the alkalinity of a given solution.
It is defined as the negative base-10 logarithm of the hydrogen or hydronium ion concentration.
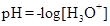
If the value of 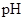 is less than
is less than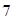 , then the solution is acidic whereas if the value of
, then the solution is acidic whereas if the value of 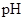 is greater than
is greater than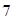 , then the solution is basic.
, then the solution is basic.
Ionic-product constant for water: It is the hydronium ion concentration times the 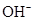 concentration present in the solution.
concentration present in the solution.
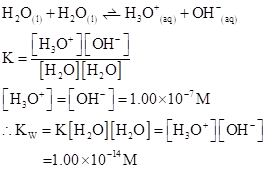
The 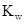 will apply to all aqueous solution.
will apply to all aqueous solution.
For acidic solution 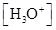 is large that is
is large that is 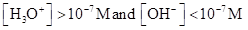
For basic solution 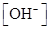 is large that is
is large that is 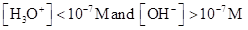
Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
Fundamentals of General, Organic, and Biological Chemistry, Books a la Carte Plus Mastering Chemistry with Pearson eText -- Access Card Package (8th Edition)
- If 0.752 moles of (NH3OH)Cl is dissolved in 1 L of water what is the pH of the solution?arrow_forwardThe H⁺ concentration in an aqueous solution at 25 °C is 4.3 × 10⁻⁶. What is [OH⁻]?arrow_forwardA solution with a pH of 6 has a ____ difference in H ion concentration than a solution with a pH of 10. If a solution has a concentration of 10^-7 OH ions, how many H ions does it have?arrow_forward
- Three buffers are made by combining a 1M solution of acetic acid and a 1M solution of sodium acetate in the ratios shown in the table below. Which of these statements is true regarding the prepared buffers? (Ka= 1.7x10-5) pH of buffer 1 < pH of buffer 2 < pH of buffer 3 pH of buffer 1 = pH of buffer 2 = pH of buffer 3 pH of buffer 1 > pH of buffer 2 > pH of buffer 3 pH of buffer 1 = pH of buffer 2 > pH of buffer 3 pH of buffer 1 > pH of buffer 2 = pH of buffer 3arrow_forwardWhat is the pH of a solution of 100 ml of 0.01 M H3PO4 and 100 ml of 0.01 M Na3PO4?arrow_forwardTo minimize the sharp pH shift that occurs when a strong acid is added to a solution, is it better to add a weak base or a strong base? Why?arrow_forward
- Calculate the pH of the solution that results following addition of 10 mL of 1 M NaOH to 40 mL of 1 M NH4Clarrow_forwardWhat is the approximate concentration of the solution in mol\kg, if 1 mol of solute particles raises the boiling point of 1 kg of solvent by 3.63 °C (3.63 K)?arrow_forwardIdentify the acid on the left and its conjugate base on the right in the following equations:(a) HOCl + H2O ↔ H3O+ + OCl-(b) HONH2 + H2O ↔ HONH3+ + OH-(c) NH4+ + H2O ↔ NH3 + H3O+(d) 2HCO3-2 ↔ H2CO3 + CO3-2 (e) PO4-3 + H2PO4- ↔ 2HPO4-2arrow_forward
- Given the following data plot of the volume of NaOH vs pH a. what does the values in 1 and 2 indicates: _____ b. Determine the molar mass of the unknown acid: _____ c. choose the identity of the acid from the choices below: _____ acetic acid, MM = 60.05 g/mole formic acid, MM = 40.03 g/mole oxalic acid = 90.03 g/molearrow_forwardWhich solution is more acidic, one with a pH of 4 or a pH of 5? What is the concentration of H+ ions in each?arrow_forwardWhat is the pH of a 0.0032 M solution of NaOH?arrow_forward
 Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning
Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning


