
For a mixture of n-butane (1) + n-pentane (2) at 25°C, what would be the predicted bubble-point pressure using Raoult’s Law if your mixture was 20% n-butane by mole? Would you expect Raoult’s Law to be a good model for this system? Why or why not?
Interpretation:
To obtain the bubble-point pressure using Raoult’s Law and to predict if the Raoult’s law be expected to be a good model for this system.
Concept Introduction:
The Antoine equation for the 1st component
Here, Antoine constants or coefficients are A, B, and C, vapor pressure is
The Antoine equation for the 2nd component
Here, vapor pressure is
The expression of system pressure using Raoult’s Law is,
Here, liquid phase mole fraction of components 1 and 2 is
The expression to obtain the vapor phase mole fraction
Here, vapor phase mole fraction of component 1 is
The expression to obtain the liquid phase mole fraction
The expression to obtain the liquid phase mole fraction
Here, liquid phase mole fraction of component 1 is
Explanation of Solution
Given information:
Temperature is
Write the Antoine equation for the 1st component
Refer Appendix E, “Antoine Coefficients”; obtain the constants A, B, and C for
Substitute
Write the Antoine equation for the 2nd component
Refer Appendix E, “Antoine Coefficients”; obtain the constants A, B, and C for
Substitute
The mixture of n-butane (1) + n-pentane is at the temperature of 25°C. Hence, consider the system pressure is the pressure of corresponding to the temperature of 25°C of steam.
Refer Appendix A-2, “Saturated steam-temperature increments”; obtain the pressure corresponding to temperature of
Here, pressure at temperature of
Write the expression to obtain the vapor phase mole fraction
It is given that the mixture is composed of 20% of n-butane by mole. It not clear that the given mole fraction is corresponded to liquid phase or vapour phase. Hence, consider that the vapour mole fraction of n-butane is,
Substitute 0.20 for
Write the expression to obtain the liquid phase mole fraction
Substitute
Write the expression to obtain the liquid phase mole fraction
Substitute
Write the expression of system pressure using Raoult’s Law.
Substitute
Similarly using excel spread sheet, calculate the values of
| 0 | 1 | 0 | 1 | 70.7 |
| 0.1 | 0.9 | 0.00131 | 0.99869 | 70.9244 |
| 0.2 | 0.8 | 0.00262 | 0.99738 | 71.1488 |
| 0.3 | 0.7 | 0.00393 | 0.99607 | 71.3732 |
| 0.4 | 0.6 | 0.00524 | 0.99476 | 71.5976 |
| 0.5 | 0.5 | 0.00655 | 0.99345 | 71.8219 |
| 0.6 | 0.4 | 0.00786 | 0.99214 | 72.0463 |
| 0.7 | 0.3 | 0.00917 | 0.99083 | 72.2707 |
| 0.8 | 0.2 | 0.01048 | 0.98952 | 72.4951 |
| 0.9 | 0.1 | 0.01179 | 0.98821 | 72.7195 |
| 1 | 0 | 0.0131 | 0.9869 | 72.9439 |
Table 1
The plot of P versus
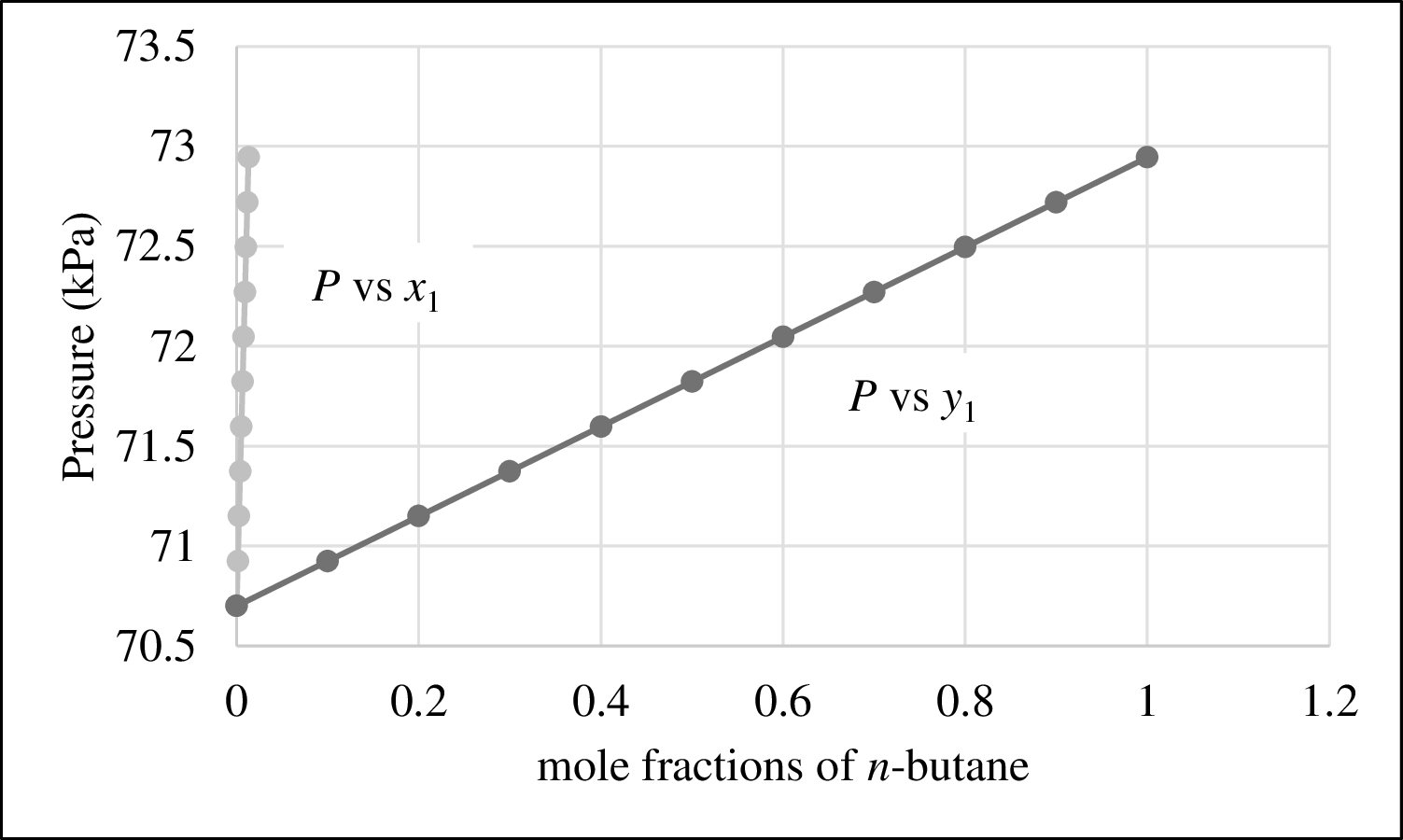
Figure 1
From Figure 1,
The bubble point curve and dew point curve are not meet anywhere. Hence Raoult’s Law is not a good model for this system and the bubble point pressure cannot be predicted.
Want to see more full solutions like this?
Chapter 10 Solutions
Fundamentals of Chemical Engineering Thermodynamics (MindTap Course List)
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





