
(a)
Interpretation:
For a binary system, the given equations are to be shown.
Concept Introduction:
For a binary system, equation
For a binary system, equation
(a)
Explanation of Solution
Given information:
Given equations to be shown are:
Here,
Rearrange and write the given equation as:
Now, take derivative of this equation with respect to
In terms of both
For excess property relation, equation (1) can be written as:
Substitute the values from equations (3) and (4) in the above equation and simplify as:
Similarly, for excess property relation, equation (2) can be written as:
Substitute the values from equations (3) and (4) in the above equation and simplify as:
Hence, the given equations are proved.
(b)
Interpretation:
The plot of
Concept Introduction:
For mixing process, equation for enthalpy is:
In terms of a third-degree polynomial equation in
Here,
(b)
Answer to Problem 11.33P
The plot of
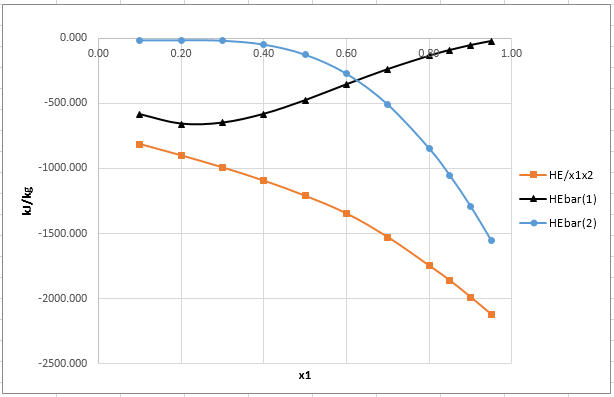
From the plot of
Explanation of Solution
Given information:
The heat of mixing data for the
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
Here,
From the equation (5), the given heat of mixing for the values of
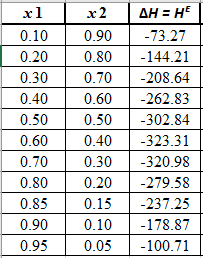
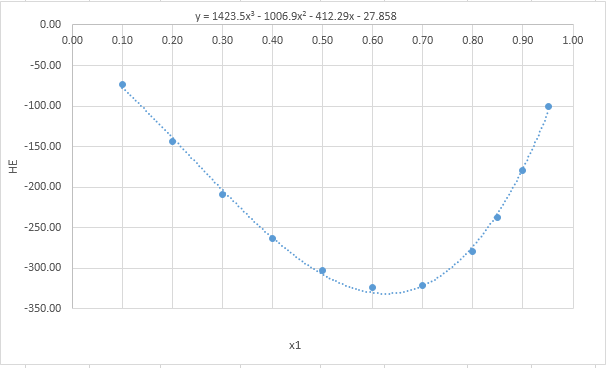
Now, plot the graph of
Now, calculate the derivative of equation (7) as:
Now, calculate the value of
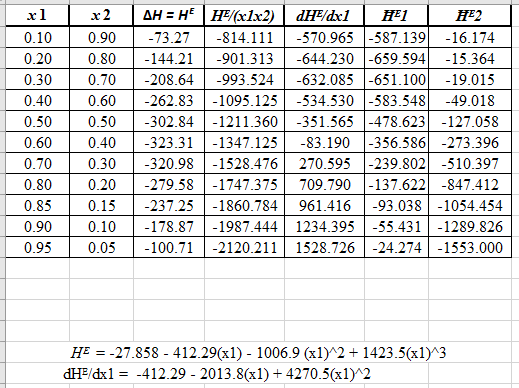
Now, plot the graph for
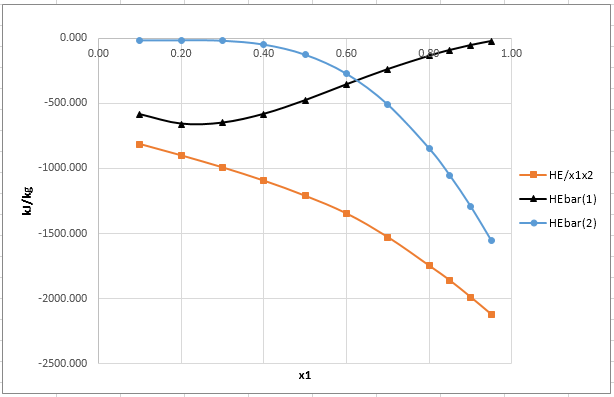
From the plot of
Want to see more full solutions like this?
Chapter 11 Solutions
GEN, ORG & BIOL CHEM: CUSTOM SSC
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





