
Concept explainers
Interpretation:
An instantaneous
Concept Introduction:
For mixing process, equation for enthalpy is:
In terms of a third-degree polynomial equation in
Here,
For a binary system, equation
For a binary system, equation
Answer to Problem 11.34P
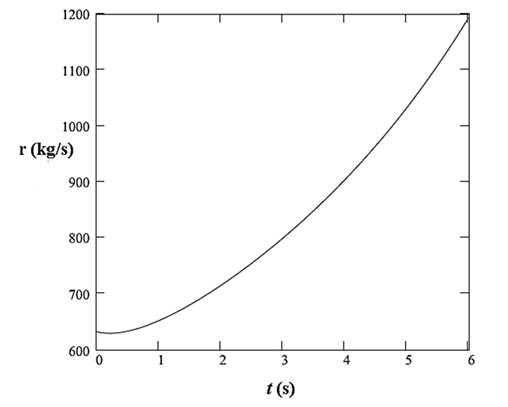
The rate of addition of sulfuric acid to the tank is defined as:
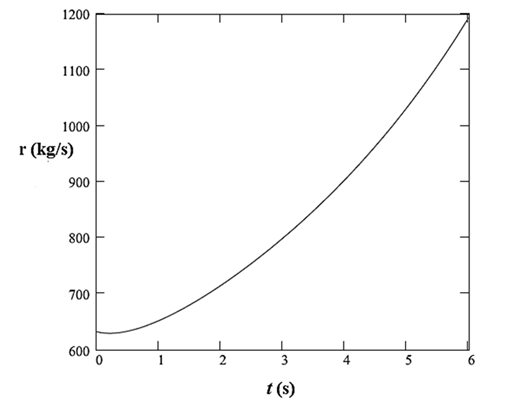
The plot of this rate with time is:
Explanation of Solution
Given information:
At
The data which may fit to the cubic equation of
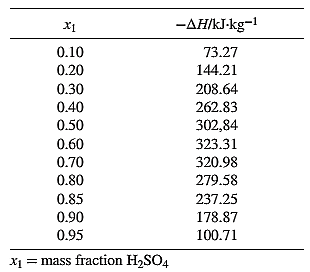
From the equation (1), the given heat of mixing for the values of
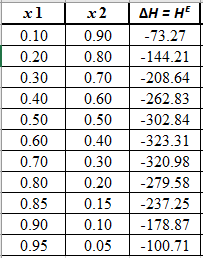
Now, calculate the value of
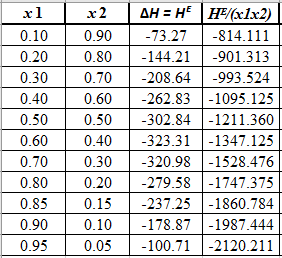
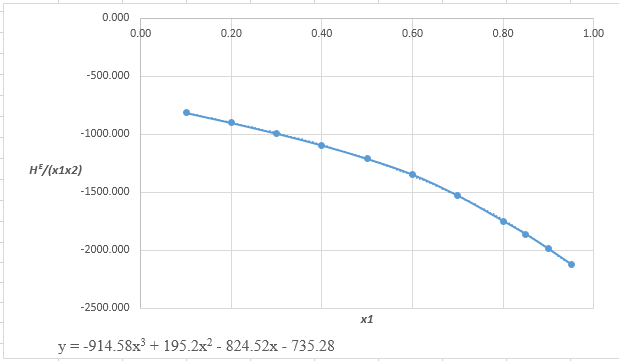
Rewrite the preceding equation in terms of
Now, calculate the derivative this equation as:
Now, calculate the value of
Now, at an instantaneous time
Apply material balance on the given process as:
Solve this equation for
Now, apply energy balance on the given process and take
Since, the temperature for the given process is taken as constant at
Using this relation, the energy balance equation now becomes:
For the overall process, the given data are:
At this
Using equation (7) and (8) and the values required, the value of
Rewrite equations (7) and (8) as a function of
It is given that the rate of heat transfer
Now, the equation for time as a function of
Take derivative of
Therefore, the rate of addition of sulfuric acid to the tank is defined as:
At
This change in enthalpy is the required heat per kg of
Now, using equations (9) and (10), plot the rate
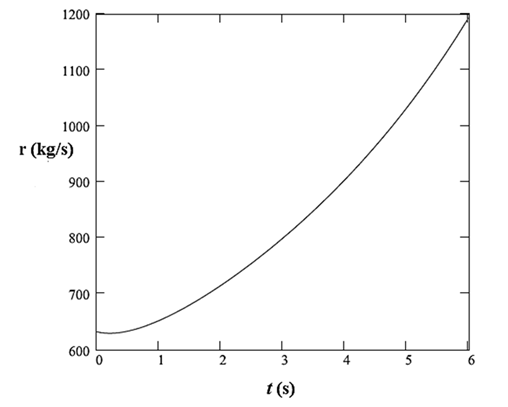
Thus, the rate of addition of sulfuric acid to the tank is defined as:
The plot of this rate with time is:
Want to see more full solutions like this?
Chapter 11 Solutions
Loose Leaf For Introduction To Chemical Engineering Thermodynamics
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





