
(a)
Interpretation:
The given reaction is to be completed with principal organic products.
Concept introduction:
The replacement or substitution of one functional group with another different functional group in any
Answer to Problem 11.60AP
The completed given reaction is shown below.
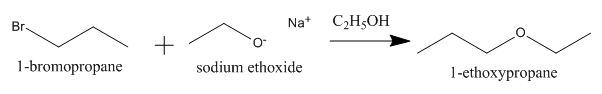
Explanation of Solution
The incomplete given reaction is shown below.
The oxygen atom of sodium ethoxide can act as a nucleophile and attack the carbon atom of

Figure 1
The completed given reaction is shown in Figure 1.
(b)
Interpretation:
The given reaction is to be completed with principal organic products.
Concept introduction:
Substitution reaction is a type of reaction in which an atom or group of atoms is replaced or substituted by another atom or group of atoms. An elimination reaction is a type of reaction in which two atoms are eliminated in the presence of solvent. Elimination reactions are usually favored in the alcoholic conditions.
Answer to Problem 11.60AP
The completed given reaction is shown below.
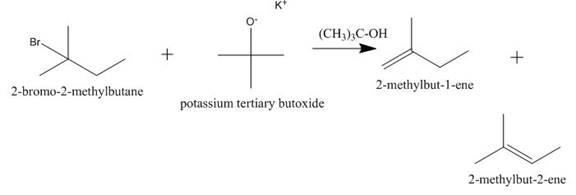
Explanation of Solution
The incomplete given reaction is shown below.
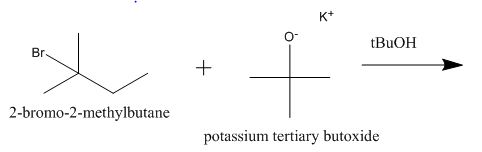
Figure 2
The given

Figure 3
The completed given reaction is shown in Figure 3.
(c)
Interpretation:
The given reaction is to be completed with principal organic products.
Concept introduction:
Oxymercuration reaction is a type of reaction in which an alkene gets converted to alcohol. The mercuric acetate is used in the reaction as a reagent. This reagent attacks the alkene to form a cyclic intermediate compound which further undergoes reduction to form alcohol.
Answer to Problem 11.60AP
The completed given reaction is shown below.
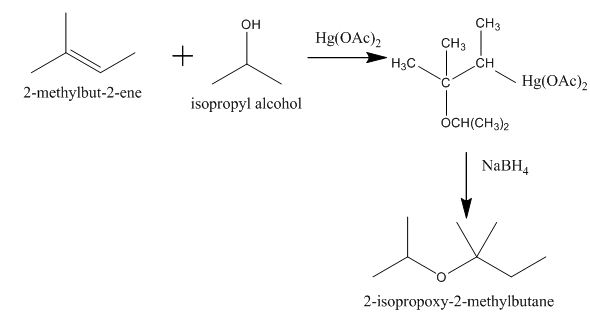
Explanation of Solution
The incomplete given reaction is shown below.
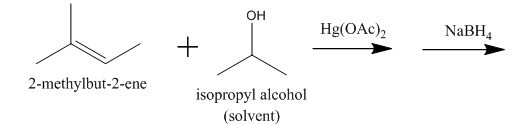
Figure 4
The mercuric acetate
The corresponding chemical reaction is shown below.

Figure 5
The completed given reaction is shown in Figure 5.
(d)
Interpretation:
The given reaction is to be completed with principal organic products. The stereochemistry of the product is to be predicted.
Concept introduction:
An
Answer to Problem 11.60AP
The completed given reaction is shown below.
Explanation of Solution
The incomplete given reaction is shown below.
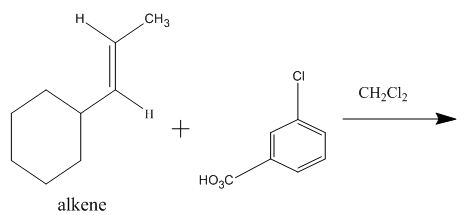
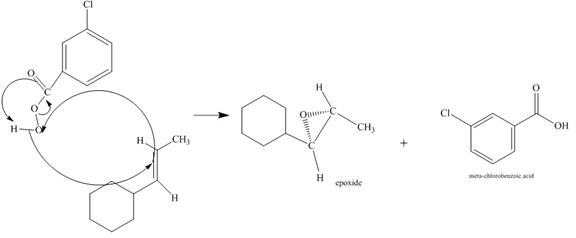
Figure 6
The alkene attacks on the peroxide linkage of the given percarboxylic acid to form an epoixde ring with elimination of carboxylic acid. The oxygen atom of the epoxide will be pointed inwards that is into the plane of the molecule. The corresponding chemical reaction is shown below.
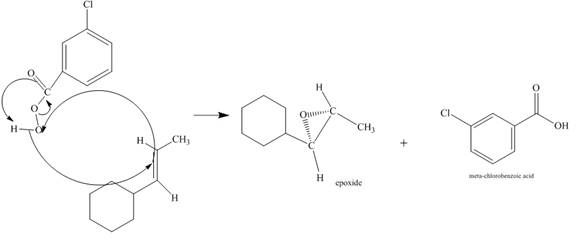
Figure 7
The completed given reaction is shown in Figure 7.
(e)
Interpretation:
The given reaction is to be completed with principal organic products.
Concept introduction:
The addition of one or more than one halogen atom in an alkene is known as halogenation reaction. The pathway of the halogenation reaction depends on the structure of the substrate. In this, the halogen is added to
The compound chromium
Answer to Problem 11.60AP
The completed given reaction is shown below.
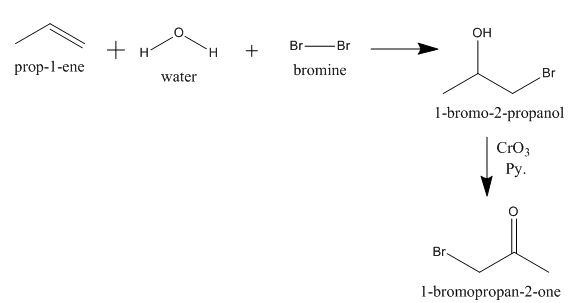
Explanation of Solution
The incomplete given reaction is shown below.
The propene molecule undergoes halohydrin reaction with bromine gas in the presence of water molecule to form bromine substituted alcohol. The alcohol gets oxidized by

Figure 8
The completed given reaction is shown in Figure 8.
(f)
Interpretation:
The given reaction is to be completed with principal organic products.
Concept introduction:
The periodic acid acts as a strong oxidizing agent. The periodic acid reacts with a vicinal
Answer to Problem 11.60AP
The completed given reaction is shown below.
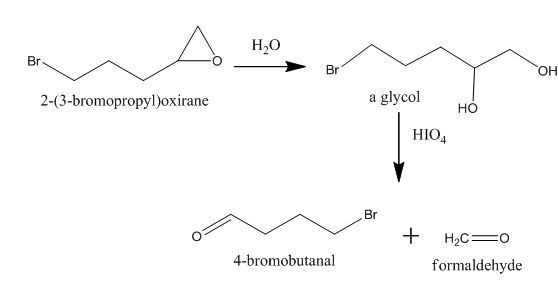
Explanation of Solution
The incomplete given reaction is shown below.
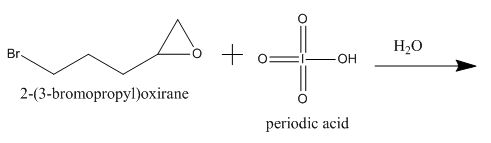
Figure 9
The epoxide is converted into gem diol after the addition of water in it. The gem diol gets converted into two different aldehydes after breaking the bond between carbon atoms attached to the hydroxyl group. The corresponding chemical reaction is shown below.

Figure 10
The completed given reaction is shown in Figure 10.
(g)
Interpretation:
The given reaction is to be completed with principal organic products. The stereochemistry of the product is to be predicted.
Concept introduction:
Potassium permanganate is a strong oxidizing agent. Potassium permanganate can oxidize an alkene into alcohol. It can also oxidize an alcohol into carbonyl compound and the carbonyl compound can be further oxidized to form a carboxylic acid.
Answer to Problem 11.60AP
The completed given reaction is shown below.
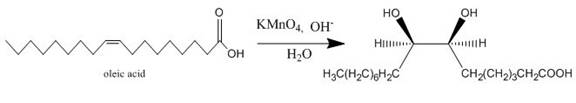
Explanation of Solution
The incomplete given reaction is shown below.
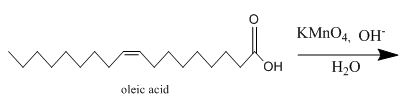
Figure 11
The oleic acid reacts with alkaline
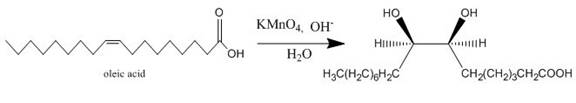
Figure 12
The completed given reaction is shown in Figure 12.
(h)
Interpretation:
The given reaction is to be completed with principal organic products. The stereochemistry of the product is to be predicted.
Concept introduction:
Epoxides undergo nucleophilic ring-opening reactions which are acid-catalyzed. If the epoxide is unsymmetrical, then the anionic nucleophile will attack the less-hindered carbon atom of the ring. If the reaction conditions are basic or neutral, then the reaction will occur at the less substituted carbon atom.
Answer to Problem 11.60AP
The completed given reaction is shown below.
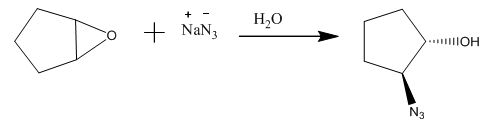
Explanation of Solution
The incomplete given reaction is shown below.
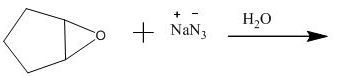
Figure 13
The compound epoxide undergoes ring-opening reaction in the presence of sodium azide. The azide ion acts a nucleophile and attacks on the carbon atom of the epoxide ring to from final compound. The corresponding chemical reaction is shown below.
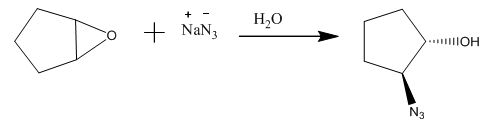
Figure 14
The completed given reaction is shown in Figure 14.
(i)
Interpretation:
The given reaction is to be completed with principal organic products.
Concept introduction:
Epoxides undergo nucleophilic ring-opening reactions which are acid-catalyzed. If the epoxide is unsymmetrical, then the anionic nucleophile will attack the less-hindered carbon atom of the ring. If the reaction conditions are acidic, then the reaction will occur at the more substituted carbon atom.
Answer to Problem 11.60AP
The completed given reaction is shown below.
Explanation of Solution
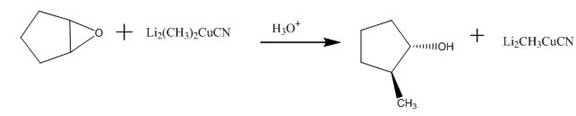
The incomplete given reaction is shown below.
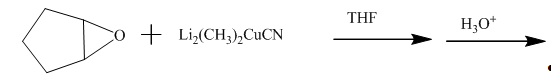
Figure 15
The ether undergoes ring-opening reaction in the presence of acid. The dilithium dimethylcyanocuprate molecule generates a nucleophile

Figure 16
The completed given reaction is shown in Figure 16.
(j)
Interpretation:
The given reaction is to be completed with principal organic products.
Concept introduction:
The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as a substitution reaction. In a nucleophilic substitution reaction, nucleophile takes the position of leaving group by attacking the electron-deficient carbon atom.
Answer to Problem 11.60AP
The completed given reaction is shown below.
Explanation of Solution
The incomplete given reaction is shown below.
The compound

Figure 17
The completed given reaction is shown in Figure 17.
(k)
Interpretation:
The given reaction is to be completed with principal organic products.
Concept introduction:
The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as a substitution reaction. In a nucleophilic substitution reaction, nucleophile takes the position of leaving group by attacking on the electron-deficient carbon atom.
Answer to Problem 11.60AP
The completed given reaction is shown below.
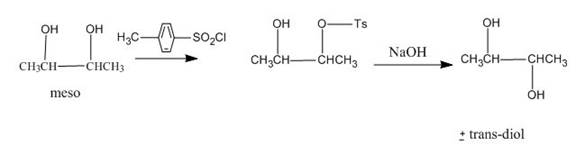
Explanation of Solution
The incomplete given reaction is shown below.
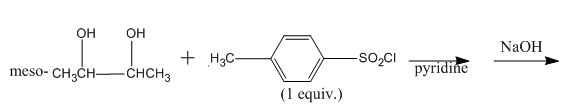
Figure 18
The replacement of the hydroxyl group is not easy. Therefore, alcohol is first converted into tosylate ester with the help of tosyl chloride. The tosylate ester group is then substituted with

Figure 19
The completed given reaction is shown in Figure 19.
Want to see more full solutions like this?
Chapter 11 Solutions
ORGANIC CHEMISTRY (LL)+ SAPLING ACC >BI
- Give a clear handwritten answer with explanation....complete the following reaction with their stereochemistry....arrow_forwardDeduce the structures of compound A,B,C and Darrow_forwardthe following reaction scheme leads to the formation of compound C. give the structure of the final product C and of the intermediate products A and B and justify, using the mechanism, the formation of the product A. Give the serereochemistry of the final product obtainedarrow_forward
- Give a clear handwritten answer...complete the following reactions with detailed explanation neededarrow_forwardExplain clearly Giving and example and mechanisms, explain the aromatic Substitution reactions.arrow_forwardGive a clear handwritten answer with explanation..complete the following reactions with their stereochemistry?arrow_forward
- Compound F may be synthesised by the method attached Draw the isomer of compound B and explain which one would be the major product and why.arrow_forwardOutline a synthesis of each of the following compounds from isopropyl alcohol. A compound prepared in one part can be used as a reactant in another. (Hint: which of the compounds shown can serve as a starting material to all others?)arrow_forwardDraw the arrow pushing mechanism for the reaction of camphor to isoborneol. For each step on the mechanism give one DETAILED sentence on what is occurring and why. Examples: "Loss of water to form a tertiary carbocation", "nucleophilic attack of ____ to increase acidity of ____", "methyl shift of ____ to form ____", etc. Thank you!arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
