
Concept explainers
(a)
Interpretation:
The formula of
Concept Introduction:
Alcohols:
Organic compounds that contain a hydroxyl group that is covalently bonded to a carbon atom
- 1) Primary alcohol: One organic group attached to hydroxyl atom.
- 2) Secondary alcohol: Two organic groups bonded to hydroxyl atom.
- 3) Tertiary alcohol: Three organic groups bonded to hydroxyl atom.
The general formula for alcohol is,
Primary alcohol:
Secondary alcohol:
Tertiary alcohol:
Phenol:
Organic compounds those in which, a hydroxyl group is attached directly to an
(a)
Answer to Problem 11D.7E
The formula of
Explanation of Solution
The structure of
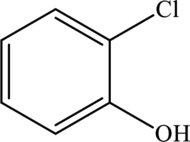
One-chlorine substituent is attached to the benzene ring in the first position and the hydroxyl group is attached to the benzene ring in second position. The formula of
(b)
Interpretation:
The formula of
Concept Introduction:
Refer to part (a).
(b)
Answer to Problem 11D.7E
The formula of
Explanation of Solution
The structure of
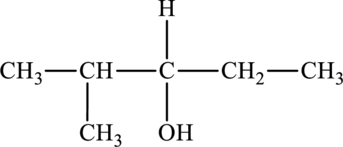
The parent chain is pentane. A hydroxyl group is present at the carbon third position and one methyl group is present in the carbon second position. The formula of
(c)
Interpretation:
The formula of
Concept Introduction:
Refer to part (a).
(c)
Answer to Problem 11D.7E
The formula of
Explanation of Solution
The structure of
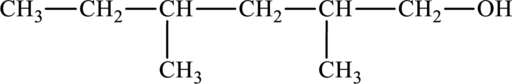
The parent chain is hexane. A hydroxyl group is present at the carbon first position and two methyl groups are present in the carbon second position and fourth position. The formula of
(d)
Interpretation:
The formula of
Concept Introduction:
Refer to part (a).
(d)
Answer to Problem 11D.7E
The formula of
Explanation of Solution
The structure of
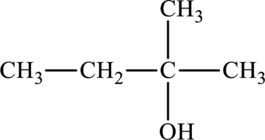
The parent chain is butane. A hydroxyl group is present at the carbon second position and one methyl group is present in the carbon second position. The formula of
Want to see more full solutions like this?
Chapter 11 Solutions
EBK CHEMICAL PRINCIPLES
- Write the chemical equation for the reaction of propanoic acid with 1-butanol (an alcohol). The formula of 1-butanol is CH-CH-CH2-CH2-OH. Which is formula of the this rule of reaction?arrow_forwardDraw and name the five cycloalkane structures of formula C5H10. Can any of these structures give rise to geometric (cis-trans) isomerism? If so, show the cis and trans stereoisomersarrow_forwardWrite a condensed structural formula for a dihydroxy compound with the formula C3H8O2.arrow_forward
- One mole of an unknown hydrocarbon, compound C, in the presence of a platinum catalyst, adds 98.9 L of hydrogen, measured at 744 mm Hg and 22 degrees C , to form a saturated alkane which contains one ring. When one mole of compound C is reacted with ozone, followed by reduction with (CH3)2S , four moles of only one product was formed, whose condensed molecular formula is CHO -CHO. Give the structure of compound C. Explain your reasoningarrow_forwardThere are 11 structures (ignoring stereoisomerism) with the formula C4H8O that have no carbon branches. Draw the structures and identify the functional groups in each.arrow_forwardGlucose, C6H12O6, contains an aldehyde group but exist predominantly in the form of the cyclic hemiacetal show below. A cyclic hemiacetal is formed when the —OH group of one carbon bonds to the carbonyl group of another carbon. Identify which carbon provides the —OH group and which provides the —CHO? Give a functional isomer of glucose and draw its structure.arrow_forward
- Identify whether oxidation or reduction is needed to interconvert alkanes, alcohols,aldehydes, ketones, and acids, and identify reagents that will accomplish the conversionarrow_forwardThe ester with the formula C8H16O2 gives an alcohol and an acid when hydrolyzed. When the alcohol is isolated and oxidized, it forms a ketone. Which of these formulas cannot be the ester?arrow_forwardName and draw structural formulas for all alkenes with the molecular formula C5H10. As you draw these alkenes, remember that cis and trans isomers are different compounds and must be counted separatelyarrow_forward
- Outline the differences in 3 physical properties between alkanes, alcohols, and carboxylicarrow_forwardI am confused as to which compound from the following: cyclhexane, ethyne, hept-2-ene, Ch2(CH2)14Ch2OH, dode-1-yne, Ch3Ch2CO2H,Propan-2-ol, Ch3(Ch2)14CO2H; is miscible in water, is an insoluble gas, is a water insosuble solid, is a wter insoluble liquid, is a water insoluble liquid- it burns clean, is a water insuloble liquid-it burns dirty, us water mscible. What do I look for to assign each compound to its coorect characterisitcs.arrow_forwardAlcohols A, B, and C all have the composition C4H10O. Molecules of alcohol A contain a branched carbon chain and can be oxidized to an aldehyde; molecules of alcohol B contain a linear carbon chain and can be oxidizedto a ketone; and molecules of alcohol C can be oxidized to neither an aldehyde nor a ketone. Write the Lewis structures of these molecules.arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning


