
Concept explainers
(a)
Interpretation:
The compound
Concept Introduction:
Organic compound is that compound in which carbon is covalently bonded to hydrogen atom or to oxygen or nitrogen atom. For example:
Inorganic compound is the one in which no carbon hydrogen
Answer to Problem 21P
Explanation of Solution
The structure of
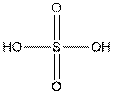
In the structure of
(b)
Interpretation:
The compound
Concept Introduction:
Organic compound is that compound in which carbon is covalently bonded to hydrogen atom or to oxygen or nitrogen atom. For example:
Inorganic compound is the one in which no carbon hydrogen
Answer to Problem 21P
Explanation of Solution
The structure of

In compound
(c)
Interpretation:
The compound
Concept Introduction:
Organic compound is that compound in which carbon is covalently bonded to hydrogen atom or to oxygen or nitrogen atom. For example:
Inorganic compound is the one in which no carbon hydrogen
Answer to Problem 21P
Explanation of Solution
The structure of
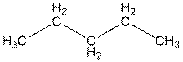
In
Want to see more full solutions like this?
Chapter 11 Solutions
GENERAL ORGANIC & BIOCHEMISTRY >ACCESS<
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





